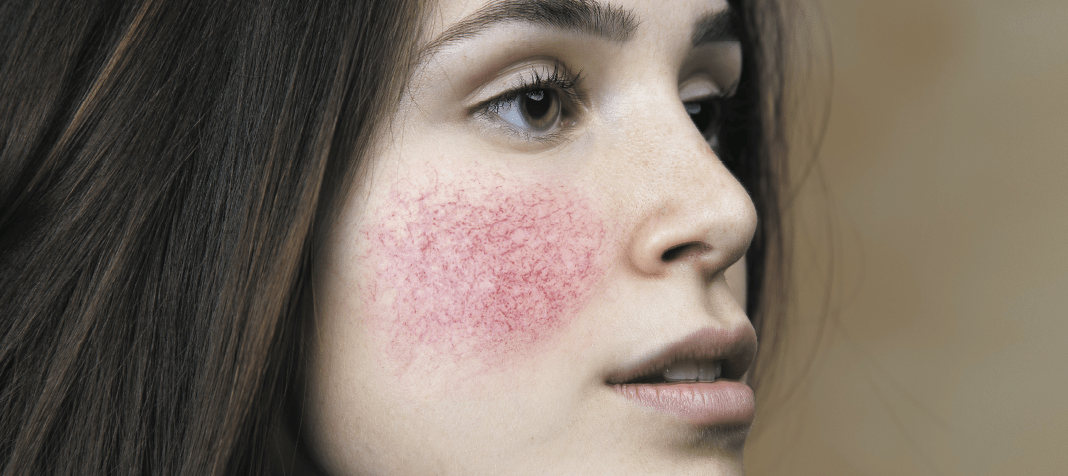
Firas Al-Niaimi, MD and Wassim Taktouk, MD discuss the links between rosacea and other systems within the body, including the gut, cardiovascular diseases and the respiratory system

Firas Al-Niaimi, MSc, MRCP, EBDV, is a world-renowned experienced professor in laser and clinical dermatology based in London and also affiliated to Aalborg University in Denmark. His private practice in London is split between Harley street and the Taktouk clinic in Knightsbridge. Wassim Taktouk, MD, of The Taktouk clinic, Knightsbridge, London
email [email protected]
Rosacea is a common inflammatory skin condition with a high prevalence among the western population and, in particular, white female women aged between 20–45 years1. While the signs and symptoms predominantly appear on the central part of the face (mid-forehead, nose, and medial cheeks), there has been increasing interest and emerging evidence linking it with other organ systems and co-morbidities beyond simply just the skin. The question one could pose is whether rosacea is a cutaneous-limited disease or one with systemic involvement or perhaps a manifestation of a wider systemic inflammation?
At the present time, the exact pathophysiology of rosacea remains unclear though it involves an interplay between an aberrant neurovascular signalling and a dysregulation of the immune system, in particular the innate system2. High levels of cathelicidins (anti-microbial peptides expressed by leukocytes and epithelial cells) have often been found in rosacea patients, in addition to kallikrein-5, matrix metalloproteinases 2 and 9 and increased mast cell infiltration1,2. It is well-established that kallikrein-5 cleaves the anti-microbial peptide cathelicidin to its more active form of LL37, which is pro-inflammatory and angiogenic (forming new vessels). Interestingly, high expression of cathelicidin has also been found in the colonic mucosa of inflammatory bowel disease patients signalling just one example of the potential link between the skin and gut system, often referred to as the ‘gut-skin axis’3.
Expanding on the above, the ‘gut-brain-skin axis’ is a term that has been existent in medical literature for a period of time, linking certain inflammatory skin conditions with stress and gut-related pathology4. In recent years, a better understanding has shed more light on rosacea and its systemic links beyond the skin. Several large case-control observational studies have shown an association between rosacea and several systemic diseases and co-morbidities, including gastrointestinal, cardiovascular, respiratory, auto-immune and neurologic disorders. Furthermore, chronic inflammation is a feature of both rosacea and several systemic co-morbidities, notably cardiometabolic disease5,6. In addition, genome-wide association studies (studying the human genome and comparing it to others with a similar disease or condition) identified (chromosomal) loci for rosacea that were also associated with several auto-immune diseases, such as diabetes mellitus, coeliac disease, and rheumatoid arthritis7.
Rosacea and gut health
Association studies linking rosacea to the gastrointestinal system have shown a link with inflammatory bowel disease and overgrowth of gut bacteria (both H.pylori and small intestinal bowel overgrowth)8. The microenvironment in the gut is increasingly linked to skin inflammation with gut dysbiosis (an alteration in the harmonious composition of the gut microbiome) playing an important role in immunity as well as controlling inflammatory signalling that may manifest in facial skin. This can either be through the mediation of inflammation through cytokines and other inflammatory mediators or through mucosal barrier compromise, often known as the leaky gut9.
Gut dysbiosis is an imbalance that is affected by multiple factors, including age, food consumption, stress and antibiotics (in anti-microbial doses). Intestinal inflammation and gut dysbiosis activate the plasma kallikrein-kinin system pathway, which is pro-inflammatory in rosacea and identified as a key step in its inflammatory cascade10. In addition, gut dysbiosis leads to mucosal barrier compromise, which, as a consequence, leads to the increase in pro-inflammatory substances circulating in the bloodstream. Control of the harmonious gut microbial environment, as well as the inflammation, can in turn have a positive effect on the control of rosacea symptoms and is increasingly being recognised as part of the overall management of rosacea through a number of interventions such as dietary modification, consumption of probiotics, as well as control and eradication of potentially pathogenic microbes such as H.pylori and small intestinal bowel overgrowth11. Gastrin-induced flushing, a symptom commonly found in rosacea patients, has also been linked to the presence of H.pylori8.In clinical practice, this can be an additional approach in the management of rosacea patients, namely those who do express gastrointestinal symptoms and may therefore have gut inflammation or bacterial overgrowth could benefit from bacterial eradication and control of inflammation. It is, therefore, of importance that rosacea patients are asked and probed for any gastrointestinal symptoms that might be associated with or contribute to their rosacea symptoms.
The skin microbiome
The skin microbiome too has increasingly been recognised to play a role in several inflammatory cutaneous diseases, including rosacea12. The skin microbiome refers to the diverse microbial population unique to each individual comprising of bacteria, viruses, fungi, and mites. Some are skin residents and act as symbionts, while others are invaders and often pathogenic. Advances in genomic sequencing research, as opposed to outdated culture-based techniques, have paved the way for a much better understanding of
the microbiome’s composition on human skin. The skin microbiome is a delicate environment affected by a number of factors, such as the skin’s acidity, temperature, lipid composition, humidity, stress, pollution, dehydration, and local skin changes such as dry or moist skin13. All these factors can alter the harmony and composition of the delicate balance of the microbiome leading in some cases to a pro-inflammatory state manifesting in cutaneous disease, with atopic dermatitis being one example.
In rosacea, the presence of the mite Demodex folliculorum and the bacteria Bacillus oleronius it carries, elicit a pro-inflammatory state through activation of pattern-recognition toll-like receptors 2 (expressed on keratinocytes and dendritic cells and also known as TLR‑2) as well as interleukin-8 and TNF-alpha with downstream activation of inflammatory pathways implicated in the clinical manifestation of rosacea symptoms14. This link is further strengthened by the observation of improvement in rosacea symptoms with anti-parasitic drugs (targeting the mites) and tetracycline-based antibiotics (primarily targeting Bacillus oleronius) with as a consequence improvement and control in the inflammation with less TLR-2 activation. TLR-2 are further activated by stress and ultraviolet radiation, the latter of which increases the production of the anti-microbial peptide cathelicidin, mostly secondary to UV-induced vitamin D3 production, a possible explanation for the low prevalence of rosacea among higher skin type individuals who tend to have lower levels of vitamin D3 with as consequence less UV-induced cathelicidin and therefore less inflammatory rosacea15. It is therefore increasingly recognised that maintaining a healthy and diverse harmonious cutaneous microbiome is of importance in the control of various inflammatory cutaneous conditions, including rosacea.
Chronic inflammation
Chronic inflammation in rosacea has also been linked to cardiometabolic risks, with case-control studies linking rosacea to a high incidence of hypertension, dyslipidaemia, obesity, and an elevated fasting glucose level16. Both rosacea and dyslipidaemia patients express low levels of a protective high-density lipoprotein-associated antioxidant called paraoxonase-1 (PON-1) as well as a relatively high inflammatory baseline level of C-reactive protein (also known as CRP), a finding observed in most cardiometabolic diseases17. Interestingly, current smokers have a somewhat protective effect on their rosacea symptoms, possibly due to the vasoconstrictive effects of smoking and worsening of rosacea symptoms has been observed in past smokers possibly due to the vasodilatory effects in the absence of smoking18. It currently remains unclear if strict control of all cardiometabolic factors and diseases can positively correlate to rosacea severity and symptoms, although increasingly, this association is being observed and clinicians may want to consider this in patients with severe or resistant-to-treat rosacea. It is well observed that patients with uncontrolled hypertension may have relatively more refractory vessel closure response with lasers and a higher relapse rate. Enquiring about any history of hypertension may therefore be of prognostic value in the laser treatment of facial vessels in rosacea patients.
Stress and mental wellbeing
Neuropsychiatric associations with rosacea have found links with depression, anxiety, migraines and Parkinson’s disease19,20. As mentioned previously, stress can worsen rosacea through stress-mediated inflammation as well as through alteration of the gut microbiome (leading to gut dysbiosis) as explained earlier through the ‘gut-brain-skin axis’. Further research is ongoing on the link between rosacea and various other systemic conditions. Small single-centre studies have shown a possible link between thyroid antibodies and hypothyroidism with rosacea but these need to be replicated in larger prospective studies21.
Conclusion
The view that rosacea is a skin-limited disease only and therefore treatment should be focused on skin is somewhat naïve and incomplete. A holistic approach to management is desirable and should take into account a thorough history taking of any systemic associations and in particular signs of chronic inflammation and gut dysbiosis. Increasing evidence is linking rosacea to a wider systemic state of chronic inflammation and its associations with particularly the gastrointestinal and cardiovascular systems may warrant some adjustments in the approach and management of rosacea patients, particularly in the presence of systemic symptoms. Clinicians should be aware of the systemic associations and co-morbidities of rosacea and take these into consideration as part of the holistic care of rosacea management.
Declaration of interest None
Figures 1 © Firas Al-Niaimi
References
- van Zuuren EJ. Rosacea. N Engl J Med. 2017;377(18):1754-1764.
- Schwab VD, Sulk M, Seeliger S, et al. Neurovascular and neuroimmune aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15:53-62.
- Kusaka S, Nishida A, Takahashi K, et al. Expression of human cathelicidin peptide LL-37 in inflammatory bowel disease. Clin Exp Immunol. 2018;191(1):96-106.
- Arck P, Handjiski B, Hagen E, et al. Is there a ‘gut-brain-skin axis’? Exp Dermatol. 2010;19(5):401-5.
- Haber R, El Gemayel M. Comorbidities in rosacea: a systematic review and update. J Am Acad Dermatol. 2018;78(4):786-792.
- Duman N, Ersoy Evans S, Atakan N. Rosacea and cardiovascular risk factors: a case control study. J Eur Acad Dermatol Venereol. 2014;28(9):1165-1169.
- Egeberg A, Hansen PR, Gislason GH, Thyssen JP. Clustering of autoimmune diseases in patients with rosacea. J Am Acad Dermatol. 2016;74(4):667-72.e1.
- Searle T, Ali FR, Carolides S, Al-Niaimi F. Rosacea and the gastrointestinal system. Australas J Dermatol. 2020;61(4):307-311.
- Kim HS. Microbiota in Rosacea. Am J Clin Dermatol. 2020;21(Suppl 1):25-35.
- Dreno B, Araviiskaia E, Berardesca E, et al. Microbiome in healthy skin, update for dermatologists. J Eur Acad Dermatol Venereol. 2016;30(12):2038–47.
- De Pessemier B, Grine L, Debaere M, et al. Gut-Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms. 2021;9(2):353.
- Holmes AD. Potential role of microorganisms in the pathogenesis of rosacea. J Am Acad Dermatol. 2013;69(6):1025–32.
- Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nat Rev Microbiol. 2018;16(3):143-155.
- Reilly N, Menezes N, Kavanagh K. Positive correlation between serum immunoreactivity to Demodex-associated Bacillus proteins and erythematotelangiectatic rosacea. Br J Dermatol. 2012;167(5):1032–6.
- Chung C, Silwal P, Kim I, et al. Vitamin D-Cathelicidin Axis: at the Crossroads between Protective Immunity and Pathological Inflammation during Infection. Immune Netw. 2020;20(2):e12.
- Searle T, Al-Niaimi F, Ali FR. Rosacea and the cardiovascular system. J Cosmet Dermatol. 2020;19(9):2182-2187.
- Hua T-C, Chung P-I, Chen Y-J, et al. Cardiovascular comorbidities in patients with rosacea: a nationwide case-control study from Taiwan. J Am Acad Dermatol. 2015;73(2):249-254.
- Li S, Cho E, Drucker AM, et al. Cigarette smoking and risk of incident rosacea in women. Am J Epidemiol. 2017;186(1):38-45.
- Woo YR, Han YJ, Kim HS, et al. Updates on the Risk of Neuropsychiatric and Gastrointestinal Comorbidities in Rosacea and Its Possible Relationship with the Gut-Brain-Skin Axis. Int J Mol Sci. 2020;21(22):8427.
- Vera N, Patel NU, Seminario-Vidal L. Rosacea Comorbidities. Dermatol Clin. 2018;36(2):115-122.
- Berksoy Hayta S, Guner R, Cam S, Akyol M. Rosacea is associated with thyroid autoimmunity: a case control study. Acta Endocrinol (Buchar). 2018 Apr-Jun;14(2):248-251.