Heinz Freier and Andreas Pachten evaluate the application, mode of action, and distinction of medical and cosmetic microneedling in Europe.
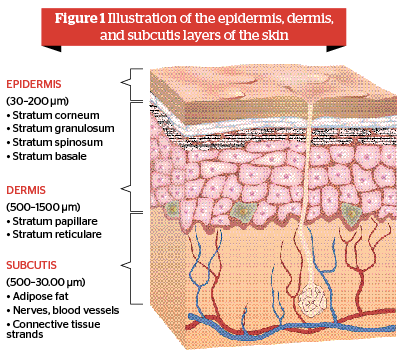
The skin consists of the epidermis, dermis, and subcutis. Skin thickness can vary in every region of the body, with the epidermis varying between 0.03 mm up to 2.0 mm in thickness depending on the skin area. Generally, the epidermis amounts to 4–8% of the skin thickness depending on the ethnic group1. The thickness of the dermis is between 0.5 mm to 1.5 mm and scientific studies describe the combined thickness of the epidermis and dermis to range between 0.5 to 2.0 mm2. Depending on the fat content the thickness of the subcutis can vary between 0.5 mm to 3 cm at the abdomen.
Microneedling instruments and devices
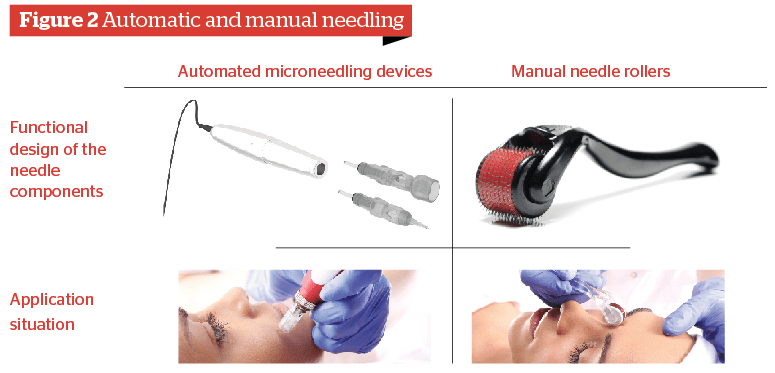
The conventional microneedling instrument is the so-called needle roller. Needle rollers are manual instruments with a handle and a cylinder, on which sharp needles with a diameter of 0.3 mm to 0.4 mm are fixed. When the instrument is rolled over the skin, the needles will penetrate the skin to different depths depending on the pressure, skin tension, and needle length, causing micro-injuries. Needle rollers create approximately 30–50 punctures per second during an appropriate application. Through repeated applications across selected skin areas approximately 250–300 punctures per cm2 are created.
Currently, the original needle roller is increasingly being replaced by automated microneedling devices. These devices resemble micropigmentation devices. Whereby, the needle cylinder is replaced by sterile single-use needle cartridges with a range of different needle configurations. Instead of a rolling motion, the approximately 0.2 to 0.4 mm thin and very sharp needles prick into the skin using a stamp motion (back and forth motion), which is created by a swing of an electric engine in the handpiece (or pen). Depending on the chosen frequency, the devices create between 50–150 stamp motions per second. Through multiple applications with the selected cartridge, several hundred punctures per second are created. Similar to the needle roller the aim is to achieve a puncture density of 250–300 punctures per cm2 of the skin.
Medical and cosmetic microneedling
The most important criterion for the definition of a medical microneedling treatment arises from the European definition of a medical or rather a medicinal treatment. The medical treatment is defined as the activities of physicians for the prevention, early diagnosis, and treatment of diseases. A disease is defined as the disruption of the function of an organ, psyche or the whole organism.
The treatment of a healthy person cannot be a medical treatment according to the above mentioned European perspective. Therefore, a medical microneedling treatment requires the treatment of a patient, which should be performed by a physician or an authorized healthcare professional.
The microneedling instrument or device used for the medical microneedling treatment requires the medical device CE marking according to European legislation. Medical devices within the scope of the Medical Device Directive 93/42/EEC (MDD) includes all instruments or devices that have been intended for use on humans for the purpose of disease treatment by the manufacturer.
In the spirit of the European regulations, a medical microneedling treatment could be performed, for example, to treat and smooth scar tissue, such as surgical scars, burn scars, and acne scars.
Microneedling treatments to the skin for the purpose of accelerated skin renewal (anti-aging), by smoothing wrinkles or skin tightening, does not count as a medical treatment in the European Union. Rather, such a treatment is defined as a cosmetic treatment. The term ‘cosmetic’ generally describes personal hygiene and beauty care. Thereby, it is focused on the preservation and the improvement of wellness and beauty of the human body. In this context, it should be mentioned that cosmetic products in accordance to the Cosmetic Regulation 1223/2009/EC can be applied on itchy skin for cosmetic purposes, for example in atopic dermatitis or psoriasis. Cosmetic creams with moisturizing and soothing ingredients can help to restore the barrier function of the skin. In this way one can prevent penetration of harmful germs and bacteria and prevent inflammation. The microneedling treatment of healthy skin for the purpose of accelerated skin renewal (anti-aging) is generally considered a cosmetic treatment.
Manufacturers determine the intended use of their devices. Therefore, it is standard practice in Europe that technically identical devices with a medical intended use to cure diseases or relieve pain according to the Medical Device Directive (MDD) (93/42/EEC) and devices without any medical intended use according to the machinery directive 2006/42/EC can be simultaneously placed on the market. For both types of equipment, conformity tests shall be carried out, which shall be verified to ensure patient safety and customer safety, respectively.
Types of microneedling
Regarding the degree of invasiveness, referring to the extent of the integrity violation of the skin (depth and intensity of needle penetration), three different types of microneedling can be distinguished:
- Non-invasive microneedling treatment
- Minimally-invasive microneedling treatment
- Invasive microneedling treatment.3
Mode of action of non-invasive microneedling
The non-invasive microneedling treatment is primarily understood to be an innovative application method for applying compounds onto the skin. These compounds can be cosmetic products, such as anti-aging lotions, as well as medical or medicinal products, including pain-relief ointments. The non-invasive microneedling treatment of the epidermal barrier improves the absorbability of the skin for the topically applied compounds. Simplified, one could say, the compound is effectively massaged into the skin by the non-invasive microneedling treatment and is, therefore, a penetration enhancer. The topical needle massage leads to cell activation in the epidermis and stimulates the metabolism and absorbability in the upper cell layers.
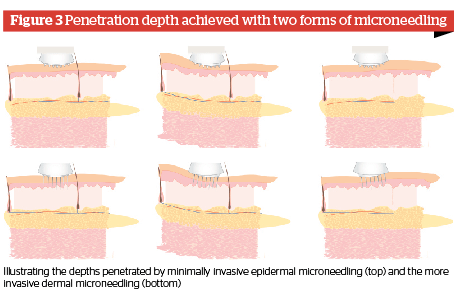
Mode of action of minimally-invasive microneedling
The minimally-invasive microneedling treatment is predominantly an application method for compounds to be applied onto the skin. The penetration depth of the needles is restricted to the epidermis during a minimally-invasive microneedling treatment. The stratum basale as the border layer to the dermis is not penetrated. Furthermore, the epidermis between the approximately 250 needles per cm2 stays intact, so that no structural impairment occurs to the epidermal and dermal skin layers. Thereby, the non- and minimally-invasive microneedling treatments comply with the requirements of the cosmetic act 1223/2009/EC for a combined application with cosmetic products, as these may only be applied to the skin topically.
To safely limit the penetration depth of the epidermis, needling devices (for example needle rollers or powered devices with needle cartridges) with very short needles are used for the minimally-invasive microneedling treatment. The needle length amounts to between 0.2 and 0.5 mm. The actual penetration depth of the needle is dependent on the number of needles per area, performance of the needle tip, needling pressure, acceleration of the needle swing, and the flexibility of the skin.
Definition of cosmetic non-invasive and minimally-invasive microneedling
A non- or minimally-invasive microneedling treatment on healthy or damaged skin in combination with a cosmetic product according to the cosmetic act 1223/2009/EC for the purpose of cosmetic skin care is regarded as a cosmetic application. As far as the authors are aware, the execution of such a treatment, for example for the purpose of accelerated skin renewal or for the purpose of moisturizing psoriasis, can be performed by a qualified beauty therapist across the EU.
Definition for medical non-invasive and minimally-invasive microneedling
A non- or minimally invasive microneedling treatment on diseased skin without, or in combination with, a topical medical compound to cure or to mitigate the pathological condition of the skin (for example pharmaceuticals according to the European pharmaceutical directive 2001/83/EC or medical devices according to the European medical device directive 93/42/EEC) is regarded as a medical application. In accordance to the nature of the skin area as well as to the medical compound the execution of the treatment is performed either by a physician or by a medical specialist delegated by a physician.
Mode of action of invasive microneedling
During invasive microneedling treatments, thousands of tight adjacent micro-lesions are introduced into the dermis by fine needles, leading to the synthesis of collagen through the initiation of the wound healing cascade4. This effect is called percutaneous collagen induction (PCI). Through PCI it is possible to effectively treat wrinkles and scars without the risk of hyper- or hypopigmentation or a dysfunction in the wound healing process due to infections.
To ensure the required penetration depth of the needle into the dermis, needling devices with longer needles and less dense needle arrangements will be used for invasive microneedling treatments compared to those used for non-invasive treatments. Depending on the performance of the needle (material, tip shape, diameter) or rather of the needle instrument, the needle used is between 0.5 and 3.0 mm in length. The actual penetration depth of the needle depends mainly on the number of needles per area, the performance of the needle tip, the needling pressure, and the acceleration of the needle swing as well as the flexibility of the skin.
During invasive microneedling treatments the epidermis is not injured across large areas but remains intact between the microlesions so that no structural weakening occurs of the epidermal and dermal skin layers. Furthermore, the expression of growth factors and proteins is induced through the procedure. These growth factors and proteins are associated with scar free healing and initiate dermal regeneration and remodeling of the skin. Through the repeated puncture of the skin surface with fine needles, the collagen synthesis will be stimulated in the area of linear scars and wrinkles5.
In all cases, an induction of collagen synthesis is achieved through adjacent sub-epidermal microlesions in the extracellular matrix. The resulting minimal bleeding activates the post-traumatic wound healing cascade, whereby the collagen structures in the scar area are replaced by a new sub-epidermal collagen matrix. In the case of microneedling, the epidermis remains undamaged in contrast to ablative procedures.
The PCI is an effective technique that offers the advantage of improving the skin appearance through stimulation of collagen production, without postoperative hyper- or hypopigmentation or fibrosis of the skin compared to the usual ablative procedures, such as laser-resurfacing or chemical peels. Furthermore, it seems that through this method the result will occur via a true skin regeneration and not through skin degeneration as caused by ablative procedures.
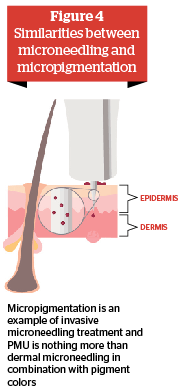
Similar observations were already made by Camirand and Doucet in 1997, as they treated hypertrophic scars with a tattoo gun and achieved very good results with a low complication rate6. It also becomes clear that the devices as well as the methodology are very similar between automatic microneedling and automated tattooing or rather during micropigmentation, for example, permanent make-up (PMU).
Comparison of microneedling and micropigmentation
Micropigmentation of the skin is an invasive microneedling treatment, which penetrates the dermis and implants pigment colors there permanently. Facial tattoos have been practiced in human society for thousands of years. In the last 20 years, PMU has been very successfully established as the modern form of facial tattooing. PMU has been a part of the initial training7 of beauty therapists for several years in Germany. In the beauty therapist bachelor directive8 issued by the German ministry of economics (BMWi) in 2015, PMU is defined as part of the examination.
The distinction between medical and cosmetic microneedling is made exclusively through the intended use of the treatment. The pigmentation of the natural areola for cosmetic purposes, for example to increase the contrast, is classified as cosmetic micropigmentation and is part of the treatment repertoire of a qualified beauty therapist. However, an areola pigmentation after a surgical breast reconstruction with post-surgical scar tissue needling is defined as medical micropigmentation. Delegated by a physician, a qualified beauty therapist often conducts medical indicated micropigmentation. A medical areola pigmentation after a plastic surgery reconstruction of the nipple completes the cosmetic reconstruction and contributes to the physical well-being of the patient. Health insurances in Germany, tend to cover these costs9 completely.
When conducting invasive microneedling procedures, the qualified beauty therapist pierces through the epidermis into the dermis with the automated needling device using needle configurations of up to 10 combined single needles with an average frequency of 100 hertz (this means up to 1000 needle punctures per second).
In Europe, the safety of the permanent implanted color is regulated through national tattoo regulations, such as German TätoV, as well as through the European tattoo resolution (resolution ResAP(2008)1). As common cosmetic micropigmentation treatments, such as PMU, apply tiny hair drawings on the eyebrows, implants of eyeliner, and full lip drawings or the improvement of the lip contours; the invasive microneedling treatment ‘micropigmentation’ illustrates that dermal (invasive) microneedling can be performed by a non-medical professional in compliance with European regulatory laws. Of course the substance, if used in combination with the needling treatment, has to be regulated in addition. Important for the distinction between cosmetic and medical micropigmentation is not the depth of the needle penetration but the intended use of the treatment.
There is no reason to proceed differently with the treatment of smoothing wrinkles via invasive microneedling. The qualification of the user and the absolute adherence to hygienic safety requirements are the main criteria for the safe and satisfactory control of the invasive methods used for the penetration of substances into healthy skin. Other invasive cosmetic treatments that follow this principle are ear piercings.
Definition for cosmetic invasive microneedling
An invasive microneedling treatment, such as those performed to accelerate skin renewal or rather wrinkle smoothing through PCI, are considered to be a cosmetic treatment. Similarly to the cosmetic micropigmentation (PMU), an execution of such treatments, for example to improve the skin appearance, can be performed by a qualified beauty therapist.
Cosmetic products according to the cosmetic regulation 1223/2009/EG may not be combined with invasive microneedling treatments in line with their intended use because these products are only meant for the application onto the skin.
Regulatory guidelines on national or European level, which would regulate the safety requirements for intradermal accessory compounds of invasive cosmetic microneedling do not exist yet. From the view of the consumers, the practitioners, and the manufacturers, it is strongly advised to close this regulatory gap in parallel to the legal regulation for tattoo agents.
The above mentioned regulatory gap is no reason for a limitation to cosmetic invasive microneedling. It is important to point out that a combination with accessory compounds is generally not required for an effective wrinkle smoothing with PCI through microneedling. If lubricants are desired for such a treatment, the usage of sterile compounds are recommended, which have been classified as uncritical, by the authors, when used during the pricking of the skin. For example, a sterile physiological saline solution could be used.
Definition of medical invasive microneedling
An invasive microneedling treatment with a medical intended use, such as scar smoothing through PCI, is considered as a medical application, which has to be executed by a physician himself or an expert delegated by the physician. As the physiological change of diseased skin tissue will be indicated here, the usage of CE market medical devices is mandatory. However, cosmetic microneedling devices require the ‘general’ CE mark, complying with the machinery directive.
The PCI-effect of medical invasive microneedling can be optimized through additional topical or transdermal application of medical products in the relevant skin area. Thereby, it refers to pharmaceuticals according to the European pharmaceutical regulation 2001/83/EC or to medical devices according to the European medical device directive 93/42/EEC, which are approved for the use in the dermis.
Conclusion
For the treatment area ‘microneedling of the skin’ it can easily be shown how a technique can be regulated for medical as well as for non-medical treatments without causing safety risks to the patient or the consumer.
Significant to the definition of when a microneedling treatment should be supervised by a physician is not the level of invasion but the intended use of the treatment. By definition, a medical care treatment requires a patient who is treated with the intention to cure or improve his illness. It is the decision of the physician if and how the microneedling treatment might be delegated to an expert, such as a qualified beauty therapist.
During the invasive microneedling treatment the epidermis is not injured across large areas but remains intact between the microlesions, so that no structural weakening occurs of the epidermal and dermal skin layers. Therefore, the treatment risk that arises from this invasive treatment technique, can be safely controlled by a qualified beauty therapist. From the view of the consumers, practitioners, and manufacturers it is, however, necessary to create clear guidelines for the safety requirements of intradermal accessory compounds for invasive microneedling on the national and European level. Cosmetic agents according to the Cosmetic Regulation 1223/2009/EC may not be combined with invasive microneedling treatments in line with their intended use because these compounds are only intended for the application on the surface of the skin.