Klaus Fritz, MD, explains how to overcome one of the limitations of using neuromodulators and enhance the duration of treatment results

It is a fact that botulinum toxin has become a staple in our portfolio of solutions due to all the indications it can treat and its amazing performance. However, it takes a few days to act, and the duration remains limited, especially compared to dermal fillers. The duration of the treatment effect is considered an unmet need within existing neuromodulators.
Manufacturers are also putting a lot of effort into further enhancing its efficacy, and we have various strategies to improve the onset and duration of botulinum toxin effects.
The best-known approach uses higher doses of botulinum toxin. Up to a certain amount of toxin helps to achieve faster outcomes and slightly longer duration of results; however, the duration is limited and can only be achieved up to a maximum of receptor saturation, beyond which there is no more increase in effectivity. Side effects, however, can also increase as the quantity of toxin increases and limits the use of ever higher doses.
For doses between >20 U–80 U of Onabotulinumtoxin A, studies1 have demonstrated a longer duration of response and higher patient-reported satisfaction versus the on-label 20-U dose with no apparent impact on safety variables. In another trial, the median duration of the IncobotulinumtoxinA effect was 175 days for the 20 U group but 215 days for the 100 U group. Also, AbobotulinumtoxinA dosed at 50, 75, 100 or 125 U tended to demonstrate elevated response rates and longer duration of effects.
Another approach3 used botulinum toxin type A reconstituted in 1% lidocaine hydrochloride with epinephrine 1:100,000. The addition of lidocaine was believed to achieve an immediate paralysing effect on the injected muscles, and the addition of epinephrine was hypothesised to minimise diffusion to adjacent muscles. In total, 58% of participants reported being more satisfied with BTX-A reconstituted in 1% lidocaine with epinephrine 1:100,000, with 85.7% of these participants reporting that the immediate results made the formulation superior; 35.7% (56/157) were indifferent, and 6.4% (10/157) reported that the modified formulation did not work better. The injection of BTX-A reconstituted in 1% lidocaine with epinephrine 1:100,000 presented no increased adverse effects (AEs), no decrease in pharmacologic potency, immediate feedback to the clinician, and higher satisfaction for the participants.
Newly approved formulations of botulinum toxin claim to be effective for longer
Alluzience® (liquid botulinum toxin type A) — claims to be effective for up to 6 months4. However, there was also a difference in the way the study was conducted compared to previous studies. In previous studies on other botulinum toxins, the evaluation was limited to less than 6 months, while with Alluzience, patients were monitored for longer, and the study documented what experienced injectors knew from other toxins: the effects can remain for longer in a small number of patients. DaxibotulinumtoxinA has been approved by the FDA for frown or glabellar lines5-6. In more than 2700 individuals with approximately 4200 treatments, there was a 6-month median duration, and some individuals maintained their results at 9 months. The results were also seen as early as 1 day after treatment. It was well tolerated and achieved high patient satisfaction. The peptide in the formulation increases the affinity of the toxin for neural membranes facilitating increased internalisation of the toxin.
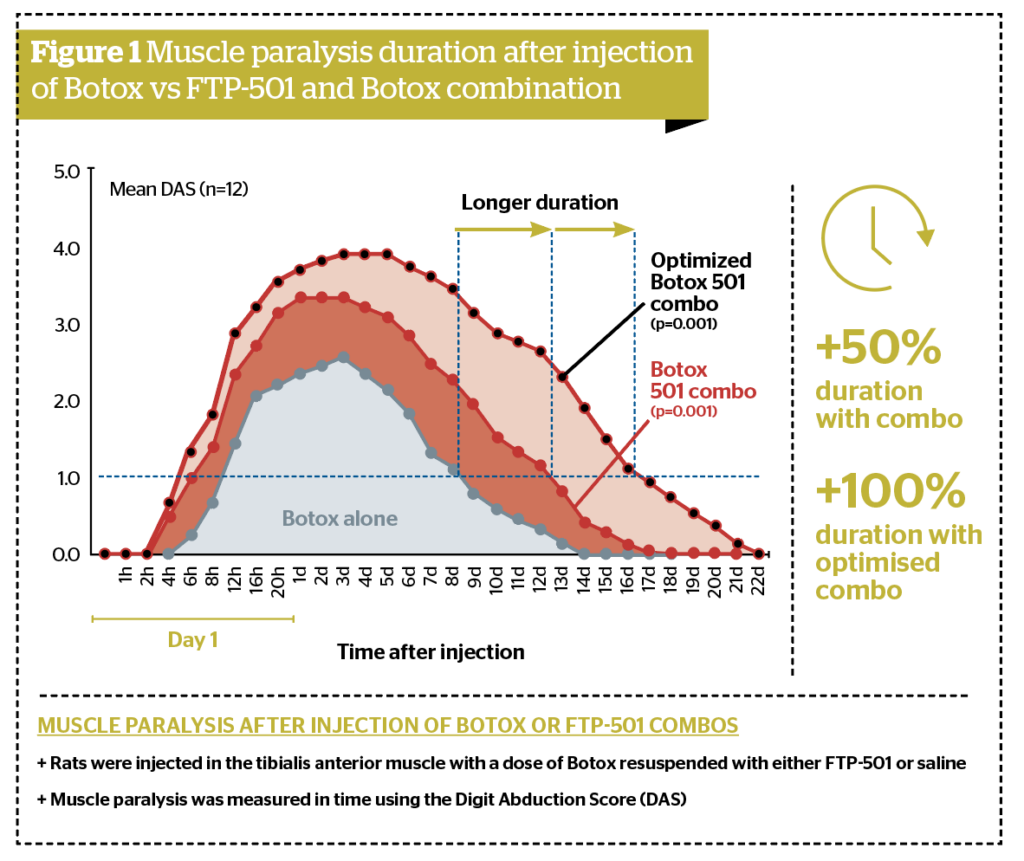
Swiss-based biotech, Fastox, is preparing for a phase I/II clinical trial with FTP-501, a proprietary formulation which, when combined with botulinum toxin products (BoNT), demonstrates faster onset, increased efficacy, and longer duration of action based on faster and higher internalisation of molecules without saturation on a cellular level. The Fastox research team discovered a unique way to enhance botulinum toxin’s activity with no effect on the fundamental properties of BoNT.
Fastox technology works on all BoNT/A tested and is proven in two validated animal models7-8.
Preclinical work has validated the use of FTP-501 with the key BoNT products marketed worldwide. It follows a completely different mechanism of action and has the potential to be more effective than high dose alone and DaxibotulinumtoxinA. The combination with any of the toxins at their standard dosage increased the duration up to 50%, and the combination at increased doses achieved a 100% longer duration. This new approach will be the next significant step in botulinum toxin development.
Due to its innovation, Fastox was awarded as the winner of two major aesthetic meeting awards: in February 2023 by the IMCAS Innovation Tank and in April 2023 at the Aesthetic Innovation Summit, Miami.
- Declaration of interest None
- Figures 1 © Dr Fritz
References
- Joseph JH, Maas C, Palm MD, Lain E, Glaser DA, Bruce S, Yoelin S, Cox SE, Fagien S, Sangha S, Maltman J, Lei X, Brin MF. Safety, Pharmacodynamic Response, and Treatment Satisfaction With OnabotulinumtoxinA 40 U, 60 U, and 80 U in Subjects With Moderate to Severe Dynamic Glabellar Lines Aesthetic Surgery Journal 2022, 1–10 /doi.org/10.1093/asj/sjac157
- Kerscher M, Fabi S, Fischer T, Gold M, Joseph J, Prager W, Rzany B, Yoelin S, Roll S, Klein G, Maas C. IncobotulinumtoxinA Demonstrates Safety and Prolonged Duration of Effect in a Dose-Ranging Study for Glabellar Lines. J Drugs Dermatol. 2021 Oct 1;20(10):1052-1060. doi: 10.36849/JDD.6377. PMID: 34636520.
- Kim A, Jung J, Pak A. Botulinum toxin type A reconstituted in lidocaine with epinephrine for facial rejuvenation: results of a participant satisfaction survey. Cutis. 2013 Jul;Suppl:13-8. PMID: 24308152.
- Ascher B, Rzany B, Kestemont P, et al. Liquid Formulation of AbobotulinumtoxinA: A 6-Month, Phase 3, Double-Blind, Randomized, Placebo Controlled Study of a Single Treatment, Ready-to Use Toxin for Moderate-to-Severe Glabellar Lines. Aesthet Surg J. 2020;40(1):93–104
- Bertucci V, Solish N, Kaufman-Janette J, Yoelin S, Shamban A, Schlessinger J, Snyder D, Gallagher C, Liu Y, Shears G, Rubio RG. DaxibotulinumtoxinA for Injection has a prolonged duration of response in the treatment of glabellar lines: Pooled data from two multicenter, randomized, double-blind, placebo-controlled, phase 3 studies (SAKURA 1 and SAKURA 2). J Am Acad Dermatol. 2020 Apr;82(4):838-845. doi: 10.1016/j.jaad.2019.06.1313. Epub 2019 Nov 29. PMID: 31791824.
- Solish N, Carruthers J, Kaufman J, Rubio RG, Gross TM, Gallagher CJ. Overview of DaxibotulinumtoxinA for Injection: A Novel Formulation of Botulinum Toxin Type A. Drugs. 2021 Dec;81(18):2091-2101. doi: 10.1007/s40265-021-01631-w. Epub 2021 Nov 17. PMID: 34787840; PMCID: PMC8648634.
- Botulinum Toxin Type A Duration Enhancement by Mu-Conotoxin CnlllC. Presentation at Toxins 6th International conference 2022 New Orleans
- Machicoane M, Onillona P, Stazib M, Tonellatob M,Crosa C, Pirazzinib M, Rossettob O, Le Doussala JM. Botulinum Toxin Type A Duration Enhancement by Mu-Conotoxin CnIIIC Toxicon Volume 214, Supplement 1, July 2022, Pages S38-S39





