With this clearance Renuvion is the only product that is FDA-cleared to improve the appearance of loose skin on the neck and chin. Renuvion’s patented technology offers a game-changing option in cosmetic surgery for neck laxity procedures.
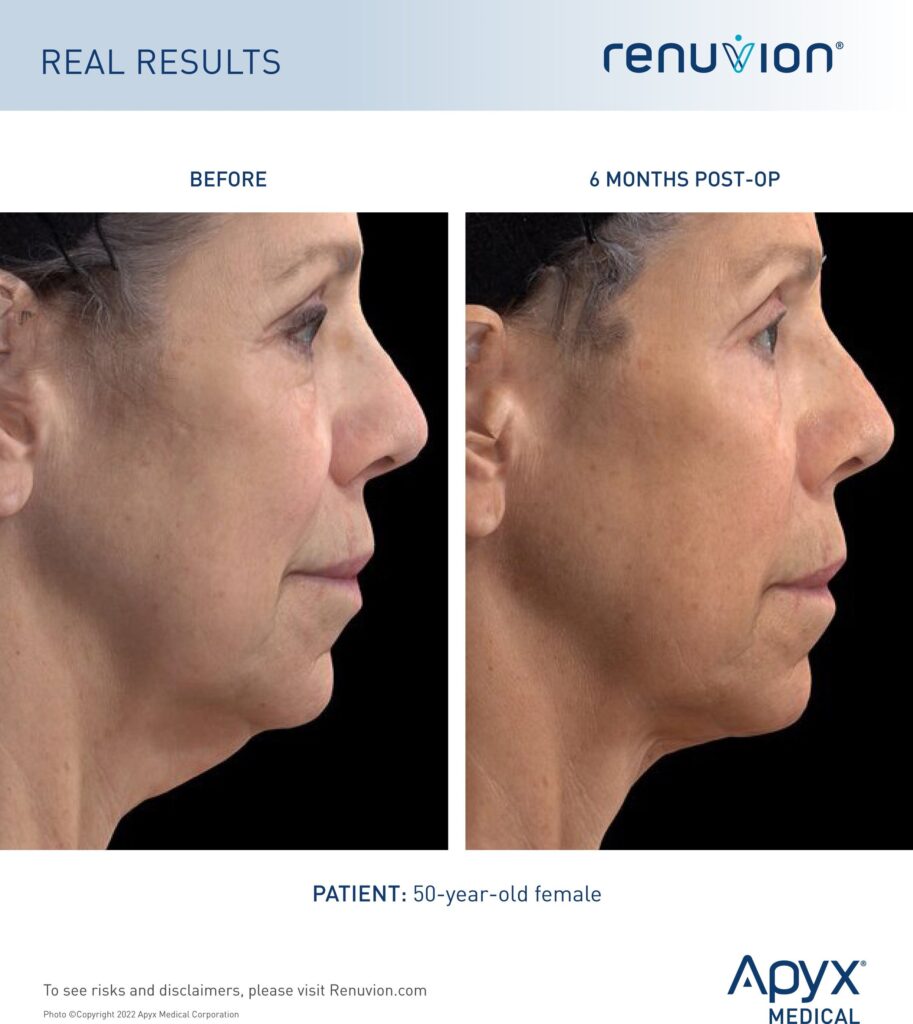
Loss of jawline definition with age is inevitable and can add years to one’s appearance. Renuvion provides a minimally-invasive and cost-effective option with minimal downtime. The results reveal a more contoured and smoother neck and jawline.
Within the fat layer beneath the skin, there is a web-like structure of collagen fibers. These fibers provide support and elasticity to the skin itself. Age, weight fluctuations, and sun exposure contribute to the breakdown of these fibers, causing the skin to sag. Surface treatments often can’t delivery energy deep enough to effectively treat these fibers, and invasive surgery can be painful and is associated with having a long downtime. The Renuvion energy is used in a minimally-invasive procedure to directly treat the collagen fibers and address the root cause of neck and chin laxity.
“We are very pleased to receive FDA clearance enabling Apyx Medical’s Renuvion technology to be used in neck and chin laxity procedures,” said Charlie Goodwin, President and Chief Executive Officer. “We’ve seen a considerable uptick in the market for these cosmetic procedures and are thrilled to be able to offer our unique technology to physicians and their patients to meet this growing demand – it’s the next generation option for neck contouring procedures.”





