Red M. Alinsod evaluates the results of his study on the effectiveness of non-invasive transcutaneous temperature controlled radiofrequency for vulvovaginal rejuvenation.
The vaginal wall predominantly consists of dense connective tissue that is heavily vascularized and through which many nerves pass, lined by a slightly keratinized, stratified squamous epithelium. The vulva, particularly the labia majora, is even more skin-like although generally more heavily vascularized and innervated than skin in most bodily regions. During vaginal delivery, stretching causes damage to the connective tissue that heals in a varying state of laxity that increases with each successive birth; the vulva is similarly affected. In addition, reductions in the quality of connective tissue due to neuroendocrine changes and age serve as contributing factors. This condition is rarely discussed in a clinical setting1–3. Other conditions such as stress incontinence and atrophic vaginitis arise in conjunction with vulvovaginal laxity, as well as natural results of delivery trauma and advancing age. An additional consequence to vulvovaginal laxity is reduced sensation during coitus, with a potential negative effect on sexual satisfaction and quality of life4–7.
The term ‘vulvovaginal laxity’, encompasses laxity of both the vaginal introitus and labia majora. Given that most people refer to the entire compound structure as ‘the vagina’ it stands to reason that ‘vaginal laxity’ and ‘vulvovaginal laxity’ will be used synonymously by some but it is important to note that, technically, ‘vaginal laxity’ does not involve the vulva specifically. Laxity of the vagina, specifically, is often referred to as pelvic organ prolapse but that term is also inaccurate because it refers to a more severe condition possibly involving vaginal and other genito-pelvic structures bulging into the vaginal canal and introitus, rather than laxity of the introitus itself8.
Only recently have alternatives appeared to fill the wide gulf between the two ends of the spectrum. The term ‘vaginal rejuvenation’ has arisen, and received a lot of attention and scrutiny within the emergence of novel modalities. Vulvovaginal rejuvenation with devices harnessing laser or radiofrequency (RF) energy (among others), as in aesthetic dermatology and plastic surgery on the face, neck, and décolleté9–10, is a fairly new concept with real potential for success. Numerous studies in aesthetic medicine have demonstrated tissue contraction and determined a therapeutically ideal temperature range (40°C to 45°C) in which neocollagenesis (via the healing cascade) is stimulated without causing unnecessary damage to the skin or integral tissue structures.
Transcutaneous temperature controlled radiofrequency (TTCRF) brings with it numerous advantages for the treatment of skin laxity14. RF is an established modality for tissue tightening via stimulation of neocollagenesis, denaturation of collagen, contraction, and activation of the healing cascade. This was shown in a histological study of RF in animal studies15. Thus, tissue temperature is modulated by controlling the power (the electrical voltage delivered to the RF electrode) in relation to tissue impedance, which raises tissue temperature in the proximity of the RF electrode. Thermistors and thermocouples within the treatment probe provide feedback to the device, which controls power to modulate energy deposition and maximize therapeutic relevancy without causing damage and minimizing the potential for patient discomfort. Unlike laser-based treatments, skin type (color or pigmentation) is not an issue with RF energy; and while it is proven effective on surface skin of the face and other body regions, RF is even more effective in tissue that is naturally moist and well hydrated, as is the vaginal and ancillary structures. Treatment is non-invasive and there is no downtime.
The treatment probe is of a proprietary design developed with expert physician input. During TTCRF treatment, the probe tip is passed back and forth slowly and with wide sweeps over the desired zone of treatment for 3 to 5 minutes, until the tissue is gradually heated to the therapeutically relevant level to induce tightening of the skin, mucosa, and fascia as well as stimulate neocollagenesis. This also promotes patient comfort and assures that the practitioner does not over-treat.
The purpose of the study is to evaluate the safety, tolerability, and clinical efficacy of TTCRF as well as anecdotally document possible ancillary beneficial effects of treatment, to promote further study.
Patients and methods
In this prospective study, 23 subjects (age range 26–58 years, mean 43.6; median vaginal births=2, mean parity 1.7; 5 menopausal, 6 perimenopausal) presented with self-described mild to moderate primary or secondary vulvovaginal laxity. Associated secondary conditions (orgasmic dysfunction, stress incontinence, atrophic vaginitis) were present in most subjects. Those who presented with mild to moderate stress urinary incontinence (n=6) were evaluated by examination showing urethral hyper-mobility, positive Valsalva, and recording incontinence episodes per day along with pad counts. Severe incontinence patients with suspected ISD and positive empty bladder stress tests were excluded. Patients who complained of clinical significant atrophic vulvovaginitis (n=8) were evaluated with symptoms of vaginal and vulvar dryness as well as painful intercourse and discomfort with their clothing.
Exclusion criteria included pelvic surgery within 5 years of study commencement, presence of major psychiatric conditions or related need for medication, chronic use of anti-inflammatory agents (including steroids) and immunosuppressants, pregnancy or planned pregnancy within the study period, recent abnormal Papnicolaou test result, presence of vulvar lesions or disease (dermatitis, human papillomavirus, herpes simplex, vulvar dystrophy), or the presence of any condition or circumstance that, in the opinion of the investigating physician, may be unsafe or otherwise interfere with the study. Informed consent was obtained from all subjects prior to commencement of the study.
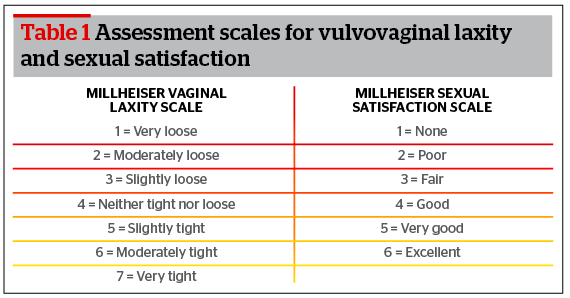
Evaluation of vulvovaginal laxity was made on a 7 point scale by both the investigating physician and patient self-assessment via VLQ11; the patient assessment data was used for analysis. A 6 point sexual satisfaction scale11 was also used by patients to rate sexual satisfaction. Table 1 delineates these scales.
Assessment occurred at baseline, 10 days after first treatment, before second treatment, before third treatment, and 30 days after the third treatment session. In addition, patients were asked to give a global assessment asking if they would recommend the procedure to a friend or family member (a 5 point scale where 1=strongly agree and 5=strongly disagree) and to rate overall satisfaction with the procedure (a 5 point scale where 1=very unsatisfied and 5=very satisfied).
Results
Of the original 23 subjects, 6 were lost to follow-up before each of the second and third treatments, reportedly due to high satisfaction with results not necessitating further treatment in the opinion of the patient. There were no burns, blisters or major complications during or after treatments, which were described as pleasant and very comfortable. All patients (n=23) received at least one treatment, with 17 undergoing a second treatment and 11 opting for a third. Average treatment time was approximately 10 minutes for the labia and 15 minutes for the vagina, totaling 25 minutes. Patients were able to resume all activity as normal, including sexual intercourse, immediately after each treatment.
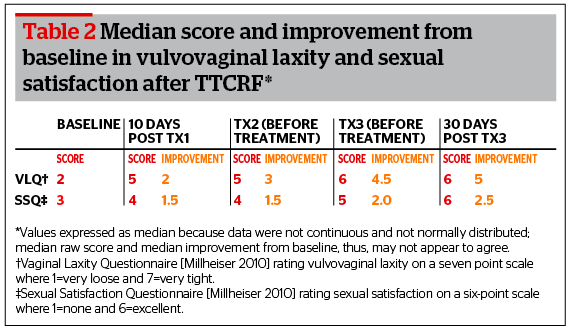
Statistically significant improvement in VLQ and SSQ scores from baseline were noted in all cases. Notably, while the patient VLQ assessment data was used for analysis, differences between patient and physician evaluation of vulvovaginal laxity were rare and overall found to be statistically insignificant (p=0.0001). Table 2 shows the median scores and level of improvement from baseline for the sake of comparison.
As seen in Table 2, the most pronounced improvement in VLQ is seen 10 days after the first treatment, with little difference noted before treatment two, suggesting that much of the result manifests rapidly. There is immediate visible correction after treatment with additional effect over time. There is some additional improvement with a second treatment, and minimal improvement with a third treatment. A similar trend can be seen for the SSQ results. It should be noted that improvement in VLQ score between sessions two and three was also statistically significant, if modest.
The overall results also correlate with patient behavior. Six patients did not return for a second treatment, and 6 opted out after two treatments, happy with results and seeing no need for further treatment.
All patients finishing a full course of treatment reported that they were very satisfied and strongly agreed that they’d recommend the procedure (scores of 5 on both global assessments).
Discussion
The tightening result is visible immediately after the first treatment and the full outcome takes a few months to fully manifest regardless of the number of sessions, but after even a single treatment the median change in the VLQ score was 3 points on a 7 point scale at the second treatment visit. Median change in SSQ score during that time was more modest but still notable (1.5). As demonstrated in those patients (n=17) electing to undergo at least one more treatment, median change in VLQ score showed that the strongest improvement occurred after the first treatment but additional, statistically significant improvement was available with a second and third session. Improvement measured by self-reported SSQ improved similarly but much less dramatically after the first session. It may be that after the first treatment pronounced improvement is perceived, with any additional improvement in sexual satisfaction appearing minimal with additional treatment, likely due to the challenge associated with self-assessment of sexual satisfaction. Small, incremental rises are less noticeable but were still reported by some patients. Overall this suggests that while one or two sessions may be satisfactory, a second session may still offer some benefit. A third may not be necessary but continued improvement may be expected. An additional study with larger populations examining protocol refinements may reveal more ideal treatment parameters and further delineate persistence of outcomes.
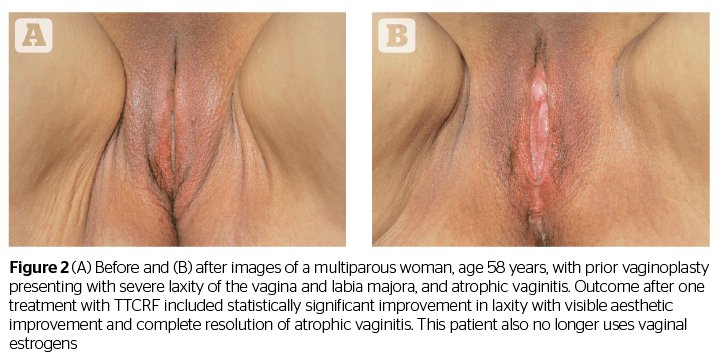
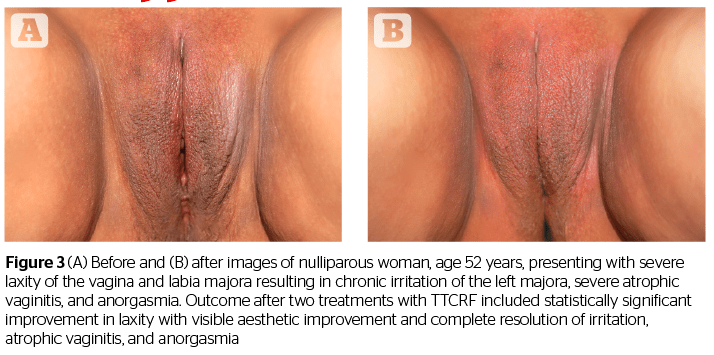
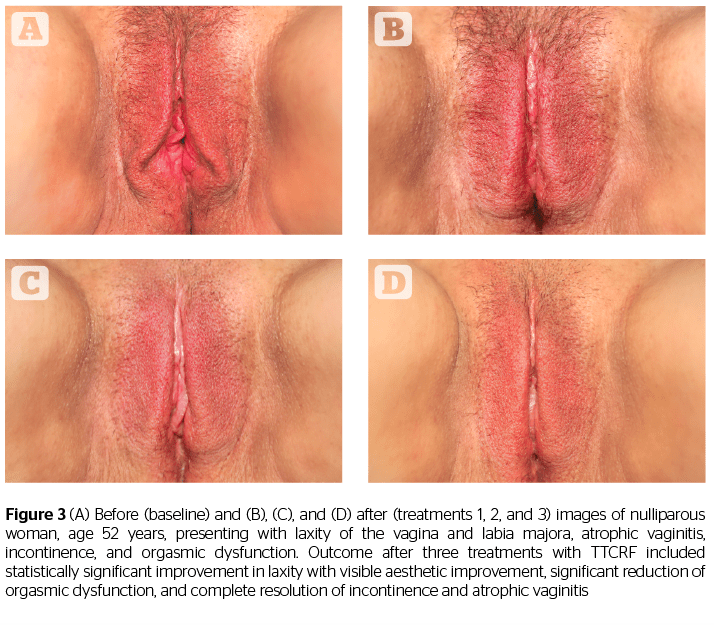
In addition to offering some confirmation of the results shared in the studies by Millheiser11 and Sekoguchi8, this investigation of TTCRF brings two additional factors into light: the treatment of the labia majora, and the use of temperature control feedback to maximize delivered energy safely. Tightening of the labia majora contributes to the positive perception of aesthetic improvement, as demonstrated by before and after photography (Figures 1, 2, and 3). Real-time feedback by which the device modulates power and thus maintains safe temperatures without cooling or anesthesia, is a boon to successful treatment and may represent a key advance in the emerging field of vulvovaginal rejuvenation. Users can rapidly treat patients with little preparation. Office time for clinician and patient is minimal, and there is no downtime or risk. Patients need not abstain from sex, nor must they interrupt normal daily activity.
Although not a specific study endpoint, anecdotal notation of improvement in related conditions such as incontinence, atrophic vaginitis, and orgasmic dysfunction was also conducted via questionnaire. Almost all patients reported marked improvement in whatever conditions they presented regardless of the number of treatments, suggesting potential significant global improvement for vulvovaginal laxity and all ancillary conditions with TTCRF treatment. All patients with incontinence (n=6) reported notable improvement (reduced or eliminated leakage or ‘dribbling’). Before treatment, these patients would suffer from 1–5 incontinence episodes per day, requiring the use of 1–5 pads each day. After treatment, five of the six patients no longer required pads and the incontinence episodes were zero. One patient continued to use pads even though the incontinence episodes were reduced by half.
All patients complaining of any level of orgasmic dysfunction, including clitorial orgasmic dysfunction (n=17, including 3 anorgasmic subjects), reported dramatic improvement (e.g. stronger, multiple, and/or more rapid achievement of orgasms with coordinated vaginal contracture during coitus). Patients who complained of lack of clitoral sensitivity and unusually long time to achieve orgasms were treated both externally on the labia/clitoral complex plus internally on the G-Spot region and entire vulvovaginal structures. This resulted in increased sensitivity of both the clitoris and vulva itself with 14 of 15 patients who complained of taking too long to achieve orgasms and a lack of clitoral sensitivity able to achieve orgasms in a third to a fifth of the time.
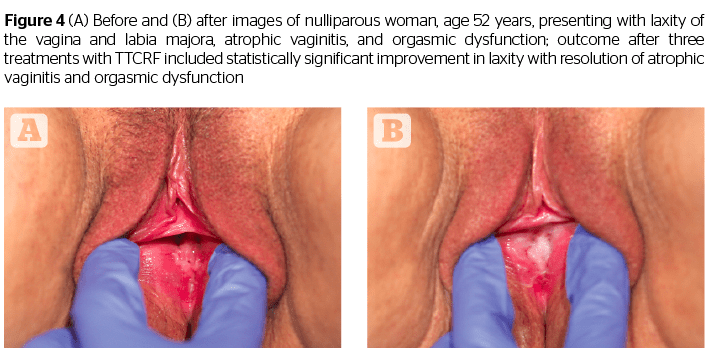
Additionally, all patients (n=8) with atrophic vaginitis reported resolution of symptoms (improved moisture/no more need for lubricants), and all menopausal women (n=5) no longer needed vaginal estrogens or lubricants. An example of a patient exhibiting successful resolution of atrophic vaginitis is seen in Figure 4. In addition, one of two patients presenting with rectocele, and both patients with cystocele, reported reduction in symptoms as well as noticeable improvement via visual pelvic examination by the investigating physician. The mechanism of action for these results is unclear but may be related to increased tightness leading to improved fascial support (pubocervical and rectovaginal fascia). This suggests the necessity of larger future studies addressing these specific issues.
While the variety of possible medical and aesthetic concerns associated with the vagina and related structures are not novel to gynecologists and urologists, increasing social acceptance of the vagina and reference to it have not only shed additional light on the prevalence, but will continue to boost demand for therapies addressing those issues. This is a boon to patients otherwise left to choose between low-efficacy pelvic floor exercises, invasive surgery, and simply not seeking treatment. Given the safety, simplicity and ease of treatment associated with TTCRF as well as the remarkable results and high patient satisfaction with virtually no risk, downtime, or discomfort, this novel therapy shows much promise in both the medical and aesthetic arenas in an increasingly accepting social climate.
Conclusion
Based on the data, TTCRF is safe, tolerable, and effective for vulvovaginal rejuvenation. Evidence strongly suggests applications in the treatment of atrophic vaginitis, orgasmic dysfunction, and stress incontinence. Further investigation via randomized, controlled trials isolating and exploring various potential indications with larger subject populations is strongly suggested.
Declaration of interest This study and preparation of this research article was funded in part by ThermiAesthetics, Inc., manufacturer of the TTCRF technology used during the investigation.
Figures 1–4 © Red Alinsod




