ABSTRACT
The use of injectable materials for soft-tissue lip augmentation has been steadily increasing both in Europe and the US over the past decade. In the early part of this century, physicians used an impressive array of augmentation products, choosing mostly between semi-permanent fillers, (collagen/hyaluronic acid derivatives) or the longer-lasting, so-called ‘permanent fillers’ — polymethylmethacrylate microspheres (PMMA), polyvinyl alcohol (PVA), highly purified forms of liquid silicone/hydrogel polymers such as Bio-Alcamid.
The hyaluronic acid (HA) based dermal fillers had a low overall incidence of long term side-effects, while many adverse outcomes, ranging from product migration to classic foreign body-type granulomatous reactions were documented from the use of these ‘longer-lasting’ lip augmenting agents. The introduction of Restylane (Galderma, Watford, UK) and other newer HA-based dermal fillers, such as Juvéderm (Allergan, Irvine, CA) and Emervel (Galderma Aesthetic & Corrective Division, Watford, UK) meant the differences in the physical and chemical characteristics of HA‑based fillers led to better patient choice and the longer duration products were mostly disbanded. This retrospective article looks at some of the adverse problems created by these more permanent fillers and their subsequent management.
Introductory Synthetic biodegradable hyaluronic acid fillers are widely used as relatively safe injectable methods for lip augmentation, but their duration is limited to only 4 months. Due to this, many physicians use alternative non-biodegradable materials like polyacrylamide gels and polyvinyl acid, to create a longer lasting ‘semi-permanent’ product. However, these fillers, once more widely used, have an increased risk of product migration, granuloma formation, and long-term adverse events. Treatment options for these types of problems include intralesional steroids, 5-fluorouracil (5-FU), anti-inflammatory and immunomodulatory drugs like minocycline or rifampicin, and surgical correction. This article documents nine cases of surgical correction for problems related to side-effects of semi-permanent fillers, Bioinblue and Bio-Alcamid (Polymekon, Brindisi, Italy) over a 3 year period.
Case report
Nine female patients in good health were referred to the author with nodular swellings in their labial area over a period of nearly 4 years. They all reported of having injections to their lips with the cosmetic filler Bioinblue or Bio-Alcamid at the site of the swelling to correct and project the labial profile. In every case, a 23 G needle was used and entrance was made 0.5 cm medial to the oral commissure with infiltration done along the vermilion border. The hemilip was injected with a maximum of 0.5 ml of Bio-Alcamid. Five of the patients were injected with Bioinblue and four with Bio-Alcamid.
Four of the patients had previously been injected with hyaluronic acid filler prior to the use of these semi-permanent type fillers. As time passed, the labial swellings were all worsening in appearance and the patients were emotionally distressed. A number of doctors had treated the patients with injections of dexamethasone (40 mg/ml) or triamcinolone (40 mg/ml) at intervals, with no resolution of the swelling. There was no relevant medical history and the patient did not have any clinical evidence of autoimmune or allergic diseases. On palpation, each patient presented with firm longitudinal swellings measuring 3 cm × 2 cm along the lines of filler implantation in each patient (Figures 1–3) . The nodules were prominent anteriorly and projected from inside the oral cavity. The patients were all willing to accept surgical correction and histopathological evaluation of their underlying problem. Surgical excisions were carried out by direct use of a size 11 scalpel blade, usually without the use of local anaesthesia, by incision and drainage of the nodules under local digital compression.
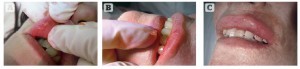
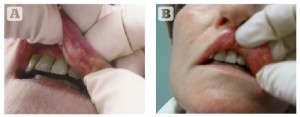
Figure 1 (A) lip nodules 2–3 months after injection with Bioinblue (Polymekon, Brindisi, Italy). (B) and (C) In this case a 23G needle was used to incise the lips and the compound was expressed
The wound was thoroughly cleaned and the vermillion tissues were approximated in some cases with 5-0 Vicryl Rapide sutures (Ethicon Endo-Surgery, Inc, Norderstedt, Germany) to achieve haemostasis. The operation sites healed well and most healed within a few days. The first excision biopsies showed no evidence of foreign body giant cells or irregular crystalline structures, and were not considered necessary for the other patients.
Discussion
Injectable fillers have become an important component of minimally invasive facial rejuvenation modalities. Their ease of use, effectiveness, low morbidity, and fast results with minimal downtime are factors that have made them popular among patients1.
The search for the ideal filling material has been ongoing for centuries. Various materials, including collagens, autologous fat, hyaluronic acids, poly-L-lactic acid, polyacrylamide, liquid injectable silicone and calcium hydroxylapatite, are among the products currently used for this indication2. Bioinblue is high purity polyvinyl alcohol (8%) and water (92%). Polyvinyl alcohol is a non-toxic substance used in medicine as a drug-carrier and a substitute for human plasma expanders. Its manufacturers claim an extended duration of cosmetic effect for up to 2 years.
Bio-Alcamid is a non resorbable polymeric material composed of 96% of apyrogenic water and 4% of an polyalkylimide group. It is sometimes referred to as an injectable endoprosthesis because its chemical and physical characteristics leave it intermediate between injectable filler and a common prosthesis3. These chemical aspects of Bio-Alcamid are responsible for a greater chemical stability of the polymer and it can sometimes be removed a long time after implantation. Its structure is quite similar to the adipose tissue4. It is a stable substance, radio transparent, highly elastic, and soluble in water. It can be extracted if necessary since it does not spread within the adjacent structures, given the fact that it gives rise to a very thin physiological capsule which isolates it from the surrounding tissues. The gel is colourless and transparent. It is supplied in packs containing two sterile 1 ml syringes for the lips (Bio-Alcamid LIPS) and one 3 ml syringe for the face (Bio-Alcamid FACE)5. It is also used medically to treat soft tissue deficits such as pectus excavatum, gluteal atrophy, acne scars as well as HIV facial lipoatrophy and Poland syndrome6–9.
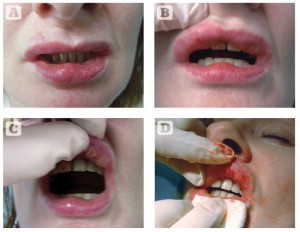
Figure 3 (A–C) BioAlcamid nodules. (D) A 23G needle was used to incise the lips and the compound was expressed
Since its initial introduction, numerous reports of adverse reactions have been reported, including infections and migration resulting in significant facial deformity10–12. Owing to this, Bio-Alcamid has been taken off the market in many countries and the original manufacture has since stopped production. In Canada, some individuals have attempted to file class action lawsuits against the company13.
Treatment options for filler complications include intralesional steroids, 5-fluorouracil (5-FU), anti-inflammatory and immunomodulatory drugs like minocycline, rifampicin or hydroxychloroquine14. Anecdotal reports also suggest some relief with the use of non-steroidal anti-inflammatory drugs (NSAIDs), antihistamines and tacrolimus14–15. In case of widespread lesions or repeated failure of conservative therapies, surgical excision is the treatment of choice16. Surgical extirpation can also allow a dermatologist to prevent the cutaneous side-effects of intradermal steroid or 5-FU injection and a histopathological confirmation can be done to rule out granuloma formation17.
Conclusion
The purpose of this retrospective case report is to demonstrate surgical removal of a semi-permanent dermal filler product when complications occur secondary to product migration, and to highlight the awareness necessary when using semi-permanent lip fillers. The ideal agent for lip augmentation should be: safe and effective; easy to obtain and administer; have a minimal risk for infection, extrusion, or migration; produce a minimal inflammatory reaction; and last for an acceptable degree of time. It should also be cost-effective, show consistency, and ultimately yield highly acceptable, positive aesthetic results.
Despite high hope for the use of Bio-Alcamid to fulfill this role, owing to its novel characteristics of apparent low incidence of risk of foreign body granulomas, the product had to be withdrawn because of other issues, relating to high incidence of infection and product migration. The long-term complications of dermal fillers can be particularly devastating, and therefore, it is important to emphasise the importance of selecting the correct substance to minimise long-term complications to patients. In the author’s opinion, official documentation of all adverse effects with dermal filler implantation, including infection and product migration should be made mandatory in order for cosmetic physicians and surgeons to make an informed choice when using injectable fillers.