Adele Sparavigna, MD discusses the results of a clinical trial using
Sunekos® Body in the mid-dermis around the knees, thighs, and arms
to treat skin roughness and laxity

Skin laxity is something everyone will face at some point in their life due to the natural ageing process. Besides ageing, there are other factors that can play a part in skin laxity, such as intrinsic and extrinsic factors, genetics, diet, lifestyle, sun exposure, stress and smoking. Sometimes following a healthy diet and workout regimen isn’t enough to target laxity in the skin. There are two vital components to the skin’s structure that will affect laxity: collagen and elastin. As we age we begin to lose these two key components and our skin begins to lose elasticity, resulting in loose or sagging skin1.
Collagen is the main structural protein found in the skin and other connective tissues throughout the body. There are at least 28 different types of collagen and we can think of them as the ‘building blocks’ of the skin.

Elastin is a protein that coils and recoils like a spring within the elastic fibres of connective tissue, accounting for the elasticity of the skin and other structures of the body. The skin depends on both collagen and elastin to remain tight to our face and body. Collagen and elastin are found in the deeper layer of the skin called the dermis, which is located right above the fatty layer which lies beneath the skin.
As we age, our body begins to stop producing collagen and elastin. Other outside factors are responsible for the destruction of these two key components in the skin. The ageing process can be accelerated by the things we are doing in our day to day lives, affecting the appearance and health of our skin.
Intrinsic factors occur internally in our body and these factors are unfortunately sometimes out of our control. As we age our skin and body begins to decline over time — genetics play a huge role in this aspect. The production of collagen and elastin slows down, resulting in changes to the skin. At 20 years of age, your body begins to produce 1% less collagen per year and the skin becomes more fragile and thin. Other internal factors, such as sweat and oil glands, begin to diminish in overall function over the years. Our sebaceous glands (oil glands) are responsible for keeping the skin naturally hydrated and supple. As the production of those glands lessens the skin becomes drier, resulting in fine lines/wrinkles.
Extrinsic ageing occurs from environmental factors including free radicals, toxins, pollutants and UV rays. In our day to day lives, we are exposed to high levels of free radicals which damage the skin and body. Antioxidants are key to preventing free radical damage so incorporating them into your diet and skin care regimen is essential.
Sunekos® Body, a new injectable registered as a CE medical device class III by Professional Dietetics, containing low molecular weight hyaluronic acid (HA) and a specific mixture of amino acids, called HY6AA, which has demonstrated to physiologically promote local neo-collagenesis and elastogenesis through fibroblasts chemotaxis migration into the injected area. The HY6AA formula has been clinically proven to be the one and only amino acid formula with the precise stoichiometric ratio to reproduce and maximise collagen and elastin2. The formula has an international patent that is applied worldwide. In an in vitro study conducted on human dermal fibroblast, the patented formula has shown effectiveness on the biosynthesis of extracellular matrix proteins, in particular elastin2. It has been demonstrated that varying the quality and quantity of amino acids in the mixtures it is possible to increase the expression, at gene and protein level, of elastin at the same time maintaining the stimulation of collagen. Sunekos® Body is derived from the expertise and experience from Sunekos® 2003, and by modifying the HY6AA formula and adapting it to the different density of the body’s skin, in order to counteract skin laxity.
The main indications of Sunekos® Body are: skin laxity on arms and legs, stretchmarks, and striae distensae in general.
To demonstrate safety and efficacy of the new Sunekos® Body a clinical trial was approved by a medical ethics committee. The primary end point of the study was to assess clinically and by non-invasive instrumental evaluations the aesthetic performance of ‘Sunekos® Body’ injected into the mid-dermis of women aged between 40–65 years with skin flaccidity of the inner knees, thighs, and arms.
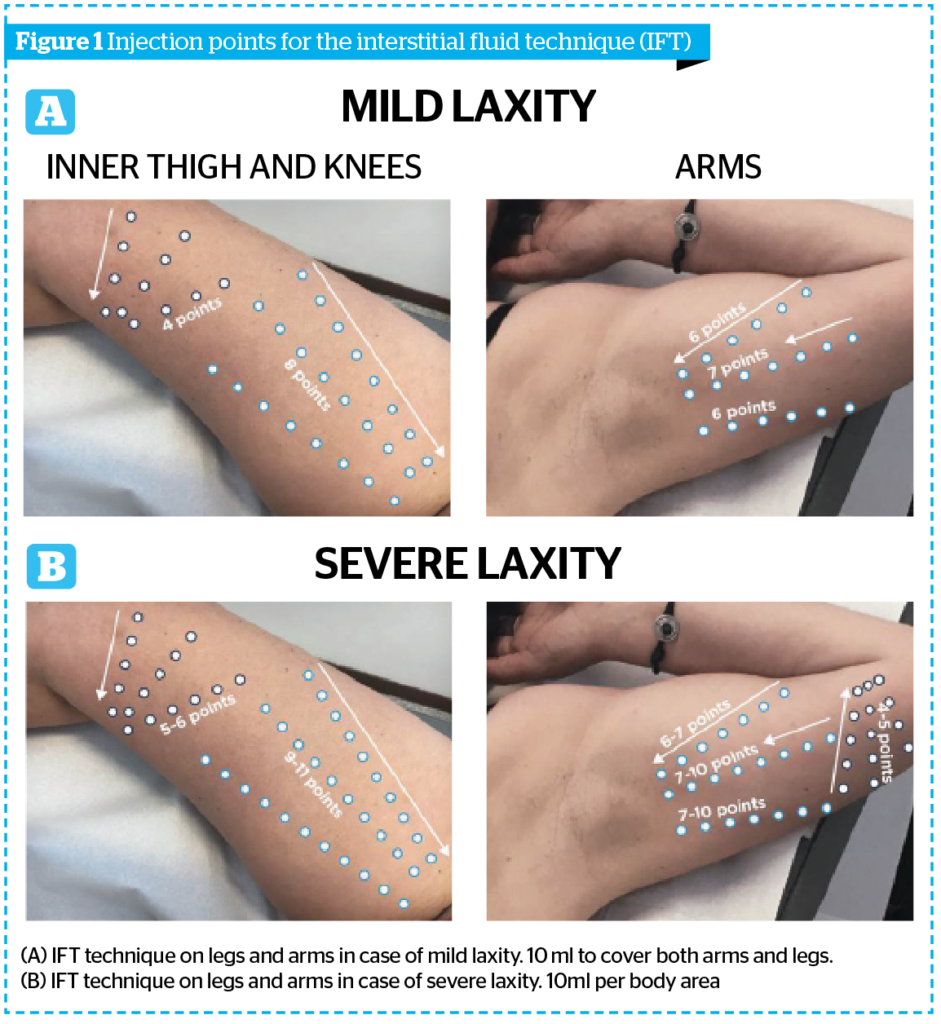
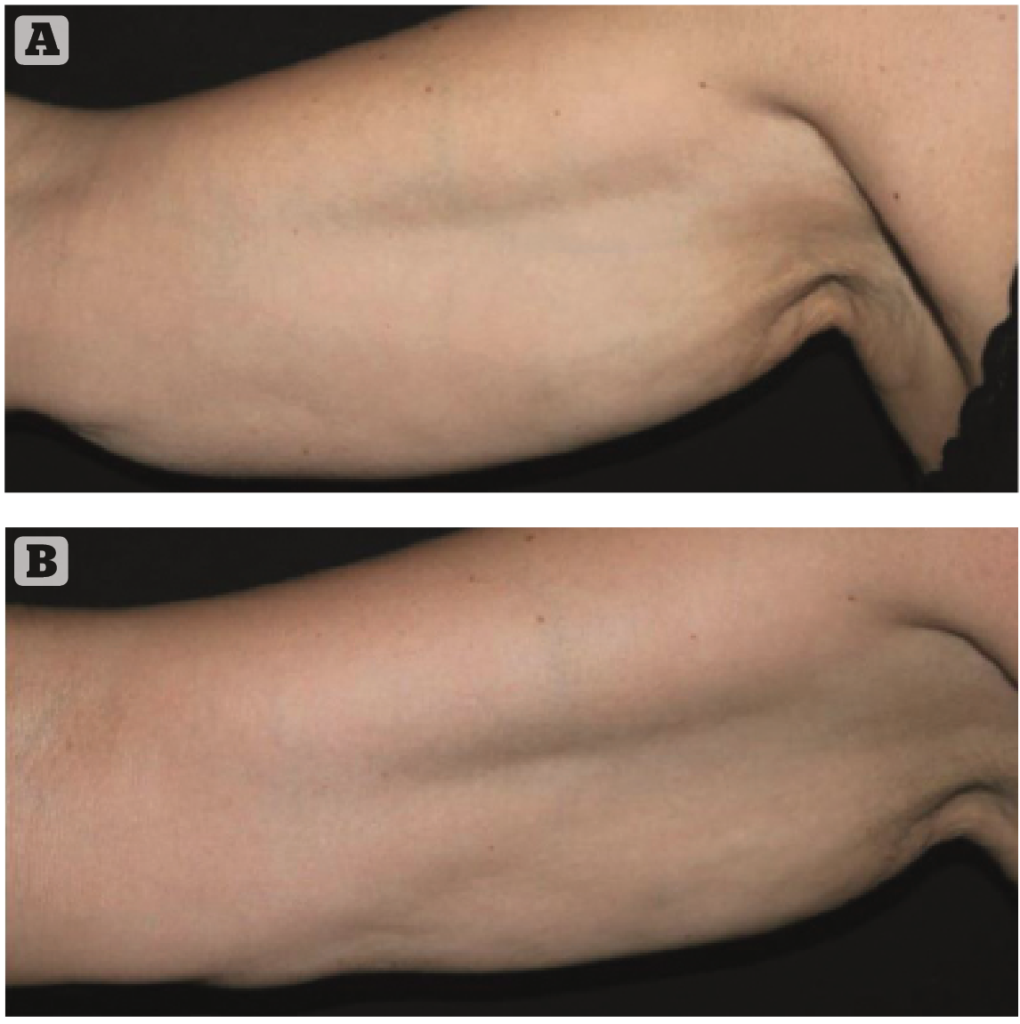
Four injective sessions with the study product were performed by a dermatologist using the Interstitial Fluid Technique (IFT). This technique involves a series of micro-wheals into the middle-deep dermis, following a triangular shape conversing towards the main lymphonodal stations of axillae, inguinal fold, and popliteal fossa; moreover, after the product injections, a slight massage with the fingertip was performed to stimulate the physiological flow of interstitial fluids (Figure 1A and 1B). The needle used was a 29G and 12 mm in the direction of the flow with a 30°– 45° inclination and retrograde linear injection. The amount injected is 0.1–0.2 ml per bolus and the series of boluses are performed at a distance of about 1.5 cm one from the other.
The trial was performed on patients with mild laxity and one 10 ml vial was used to cover both areas of the legs and arms.
In case of severe laxity, it is more effective to use one 10 ml vial on a single area (internal data, not reported in the clinical trial).
The secondary objective of this study was to evaluate the product tolerance, both by investigator and volunteers, and also efficacy by the volunteers through a self-assessment questionnaire.
The study protocol and appendices were submitted to an Independent Ethic Committee and obtained its I.E.C. approval.
Protocol
The open clinical trial was conducted by one centre under dermatological control.
The study was conducted on 24 female subjects, age range 40–65 years (mean = 52), whose informed consent had been obtained.
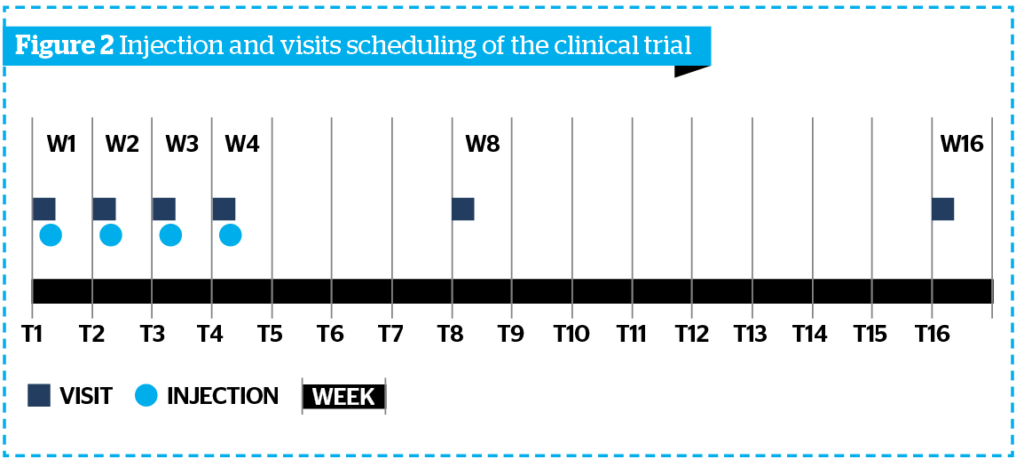
Sunekos Body was injected four times at intervals of 7 days each, while the visits were performed as described in Figure 2.
The assessment of the aesthetic results was established through the use of visual and objective assessments and supported by photographic documentation.
All clinical and instrumental evaluations were performed at T0, T8, and T16 on the knees, thighs and arms, mono-laterally on the right or left side according to a randomization list defined by the investigator before the subjects’ inclusion.
Results
The long term activity of the test product was expressed in absolute values versus baseline (T0) and in comparison with the results obtained at T8 and T16 (2 and 4 months after the first injection).
The data processing and statistical analysis were performed as follows:
- Clinical data: Friedman test followed, in case of statistically significant result, by Holm-Sidak Adjusted test.
- Instrumental data: non-parametric test (Friedman test), when the normality hypothesis was rejected by the Shapiro-Wilk normality test (threshold at 5%) or parametric test (Anova test for repeated measures), when the normality hypothesis was confirmed, followed in case of statistically significant result by Holm-Sidak Adjusted test.
Efficacy evaluation
Clinical assessment
Obtained results highlighted:
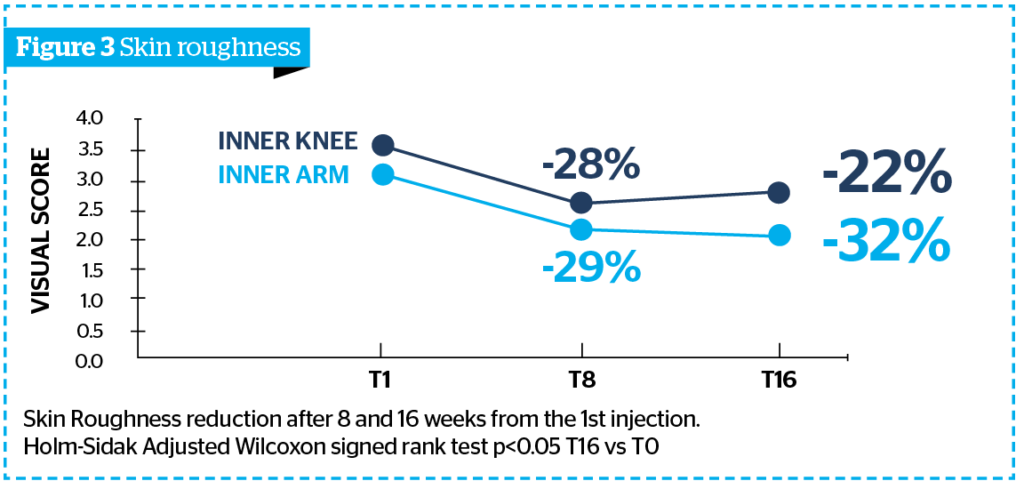
- Figure 3 shows a statistically significant inner arm and knee skin roughness reduction respectively of 32% and 22% at T16 vs T0, corresponding to a reduction of the clinical score of at least 1 grade, according to DERMING reference photographic scales on 83% of subjects.
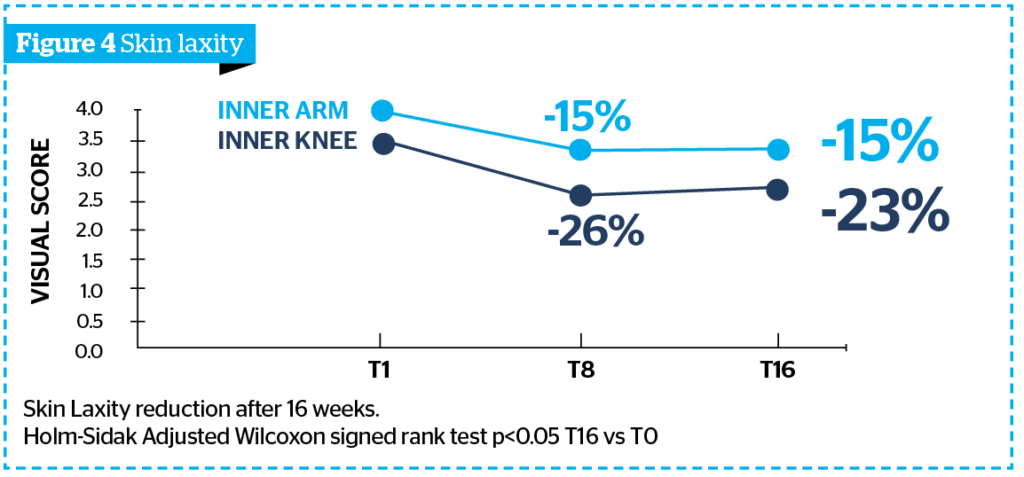
- Figure 4 shows a statistically significant skin laxity reduction vs T0 of 23% on the inner knee and of 15% on the inner arm vs T0, corresponding to a reduction of the visual score of at least 1 grade, according to DERMING reference photographic scales, respectively on 78% and 61% of volunteers.
These reduction percentages are comparable and not statistically different from the ones obtained at T8, a sign of an important and visible long lasting aesthetic performance of Sunekos® Body on the inner arm and knee.
These results are confirmed in Figure 5.
Instrumental evaluations
Skin density (profilometry)
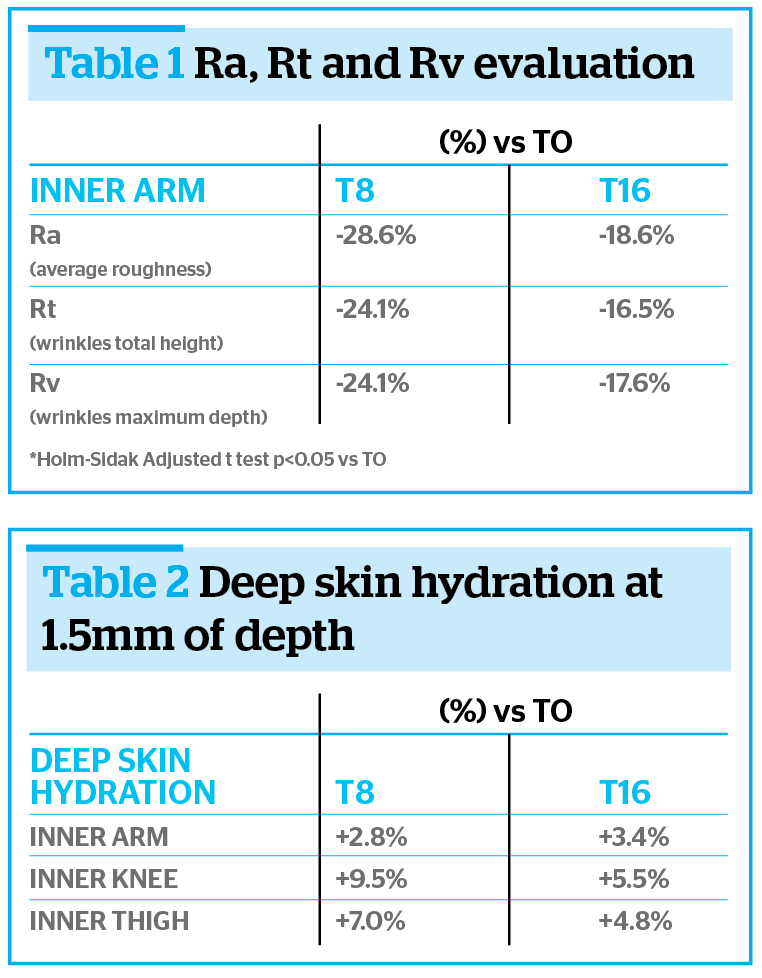
A little skin area of about 7 cm2 at level of inner arm (always the same area for each subject and at each study time) was pinched, in standardized conditions, using a specific device and a picture of the obtained skin profile was taken by Primos compact portable (GFMesstechnik) equipment. Skin density was determined by measuring of Ra, Rt, and Rv profilometric parameters, which represent respectively the average roughness, the total height and the maximum depth of the analysed skin profile. Table 1 shows the improvements in percentage at T8 and T16 vs T0.
T16 results confirmed the important and significant reduction of all profilometric parameters already highlighted at T8. The long-term evaluation shows that the skin of the inner arm is less wrinkled and more re-densified even after 16 weeks from the first treatment.
Skin hydration
Measurements of skin hydration were performed on deep skin layers at 1.5 mm of depth by MoistureMeterD and reported in Table 2.
Despite the seasonality in which the test was performed, where the prolonged exposure to the strong UV-radiation was counteracting skin hydration, the obtained results confirmed the improvement of deep skin hydration, sign of a long lasting bio-revitalizing activity of the tested product.
Efficacy evaluation by the volunteers
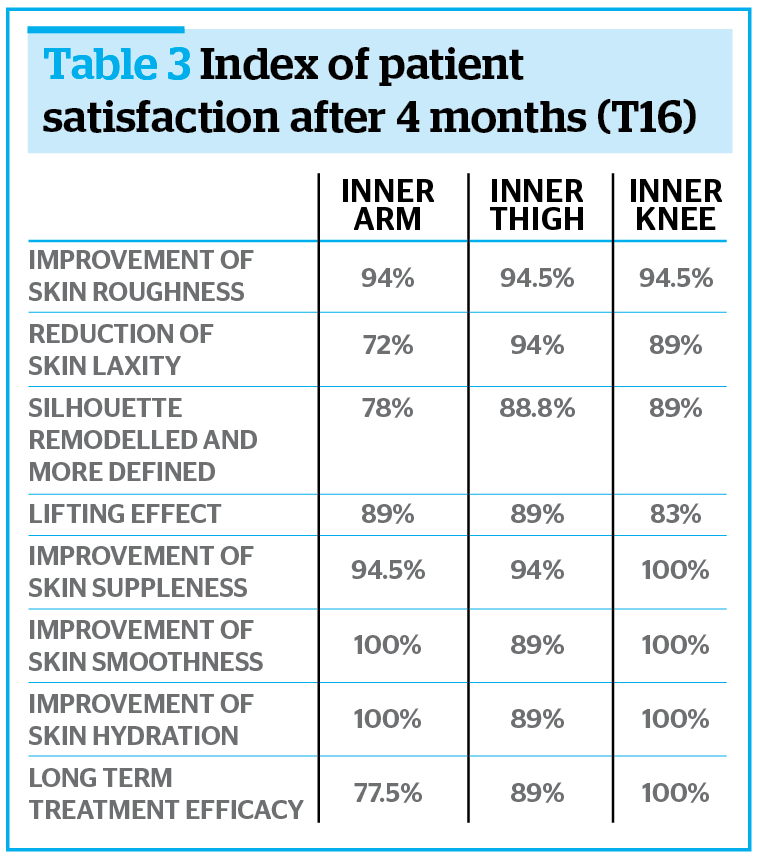
Each volunteer filled in a questionnaire regarding the perception of efficacy of the study treatment over skin laxity and roughness of the treated areas, skin hydration, skin lifting, contour redefinition/remodeling and treatment tolerance.
The long term efficacy of the study treatment was also confirmed by the high score of the index of satisfaction; in particular the maintenance of the aesthetic performance on the knees generally achieved the most positive scores (Table 3).

Conclusions
The results confirm the long lasting efficacy of Sunekos® Body injected by the Interstitial Fluid Technique (IFT) to correct body skin flaccidity and roughness.
It was observed the anti-roughness activity, as well as the re-densifying and lifting effect are still significantly present 16 weeks after the first injection; moreover, T16 has no significant difference in comparison with T8 results for any treated area.
Considering the seasonality in which the test was carried out, impacted by UV-radiation exposition, these results are even more significant and interesting.
Declaration of interest Derming received a grant from Professional Dietetics for conducting this research
Figures 1–5 © Dr Adele Sparavigna redrawn by Prime Journal
Tables 1–3 © Dr Adele Sparavigna redrawn by Prime Journal
REFERENCES
- Jones D.B. et al. Skin Laxity in Post-Weight Loss Upper Arm Body Contouring Procedures. Bariatric Times. 2016; 13(12):10-14
- De Servi B., Orlandini A., Caviola E. and Meloni M. Amino acids andhyaluronic acid mixtures differentially regulate extra cellular matrix genes in cultured human fibroblast. J Biol. Regul & Homeost. Agents, Vol. 32, no. 3, 517-527, 2018
- Sparavigna A., Orlandini A. Efficacy and Tolerance of an Injectable Medical Device Containing Hyaluronic Acid and Amino acids: A Monocentric Six-Month Open-Label Evaluation. J Clin Trials volume 7, issue 4, 1-6, 2017