Isabelle Rousseaux and Lakhdar Belhaouari explain how a thorough assessment, knowledge of the anatomy and consideration of contraindications can help avoid filler complications as well as the methods involved to treat problems when they arise
THE DEMAND FOR aesthetic treatment procedures is increasing greatly, and along with the growing number of practising doctors and patients treated, the number of complications is also rising. Filler injections are among the most common aesthetic treatments and serious complications are very rare1–3. Moreover, most complications are not actually side-effects of the products but are caused by technical errors, such as the use of an inappropriate injection technique and/or product, errors in the choice of the injection site, poor treatment indications, and a lack of consideration of contraindications.
Prevention: The initial consultation
A subjective assessment is essential to screen for contraindications and to establish the aims of the treatment with the patient4.
The patient must be questioned regarding his or her general medical history. Specific questions should then be asked regarding the following circumstances which constitute absolute contraindications:
- The previous injection of a permanent filler in the site to be treated (even if the implant has since been surgically removed, as small fragments may remain). If the patient does not know what was injected, it is best to avoid re-injection
- Generalised scleroderma or sarcoidosis
- Active, unstable auto-immune disease
- History of hypersensitivity to hyaluronic acid or one of its excipients
- Children (except for reconstructions, with parental consent)
- Hypercoagulability and high-platelet count
- Immunosuppressive medication or interferon5
- Do not inject hypertrophic or keloid scars.
Other situations constitute temporary contraindications:
- Ongoing orthodontic treatment, poor oral hygiene
- Pregnancy or breastfeeding
- Acute infectious disease
- All serious unstabilised conditions.
As a general rule, it is essential to be aware of all aesthetic treatments previously carried out on the site to be injected (permanent and semi-permanent fillers, unknown products, threads and implants). It is also important to note all long-term medication taken during the previous 10 days that may either be a contraindication to treatment or may reveal a pathology that contraindicates filler injection.
Untreated epilepsy, uncontrolled diabetes, porphyria, history of herpetic infection around the face, anticoagulation or antiplatelet therapy prescribed for serious pathology, which cannot be interrupted; pathologies which cause coagulation disorders, and low platelet count are relative contraindications that require, at the very least, specific precautions to be taken during the procedure. It is always preferable to only inject patients who are in good health or whose pathologies are well controlled.
Patients who tend to form keloid or hypertrophic scars are potentially predisposed to granulomas. This type of history should be documented, and the risk should be clearly explained to the patient6.
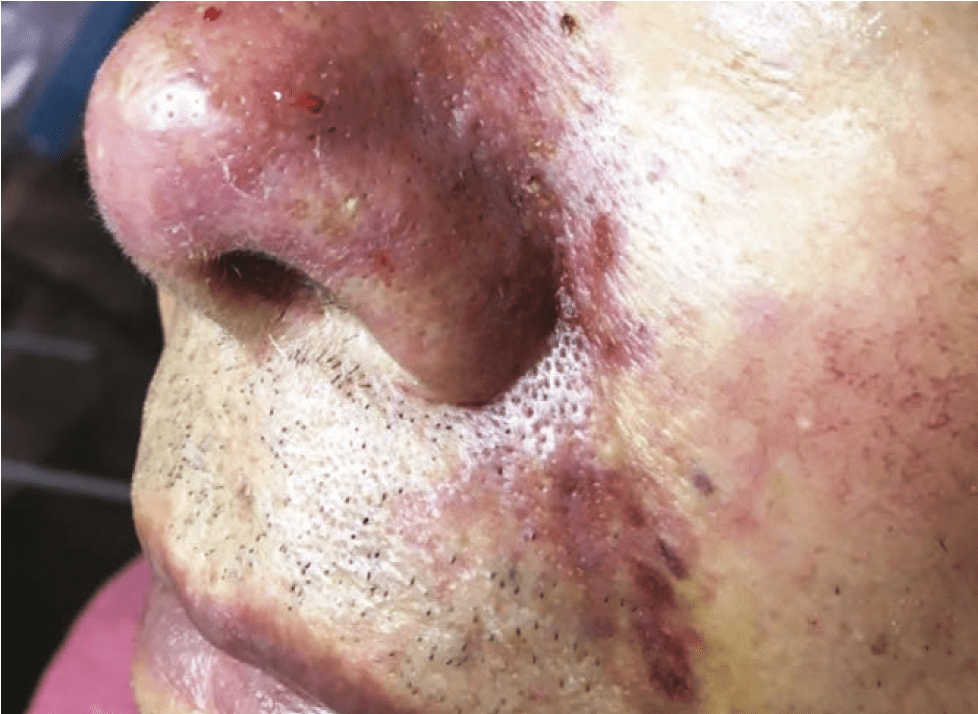
Figure 1 Vascular embolus with starting necrosis
In patients with a history of severe anaphylactic-type allergies, it can be useful to carry out a pre-treatment test as a measure of precaution. This involves two subcutaneous injections of hyaluronic acid in the forearm, 2 weeks apart. The result should be assessed 6 weeks after the second injection.
In some cases, patients who have been refused treatment by a well-trained colleague because they present a contraindication, deliberately omit to inform of the disease or situation (particularly previous injections of non-resorbable products) in order to receive further injections. All patients must, therefore, be informed of the possibility of serious complications associated with some of the previously mentioned contraindications.
It is also prudent to formalise that there are no contraindications or risk factors present by asking the patient to sign an informed consent form that includes a list of the contraindications.
Initial assessment
observation and palpation
The initial examination is useful to screen for certain contraindications and to determine the indications for treatment.
The patient’s face should be inspected to determine the topography of the problems to treat, any grooves caused by skin slackening, expression lines, and to propose the most suitable treatment. Any cutaneous inflammation or active infectious process (such as acne or herpes) can also be detected.
The face should also be palpated to identify bony contours as well as any permanent implants that the patient may not have mentioned. It is also important to check the mouth mucosa for scars or implants before carrying out injections in the lips.
The examination is also a useful time to explain the treatments proposed to the patient, including the final effect, the time it will take for the effect to be noticeable and its duration, as well as any risks. It is essential to ensure that the patient’s wishes have been well understood and equally, that the patient has understood what can and cannot be done, and the risks inherent to the injection. A well-informed patient is easier to manage should a problem occur4.
It is highly advised to photograph the patient before and after the injections, not only as an objective measure of the effect of the treatment but also for medicolegal reasons4.
Patients who tend to form keloid or hypertrophic scars are potentially predisposed to granulomas. This type of history should be documented, and the risk should be clearly explained to the patient.
The injection procedure
As long as there are no contraindications, long-term anticoagulant/antiplatelet treatments should be temporarily stopped at least 2–3 days before injection, or 10 days before in the case of antiplatelet treatments such as aspirin or NSAIDs. The cardiologist’s opinion should be sought before interrupting any treatment.
Treatment should be delayed until at least 2 weeks after any ongoing dental treatments.
The patient must be comfortable and as relaxed as possible — stress and pain can increase the risk of unwanted effects.
If a combined procedure is planned, it should be carried out over several sessions. Filler injections should be delayed until complete resolution of the inflammation following any laser treatments, chemical peel or dermabrasion. Filler injections and botulinum toxin injections should not be carried out in the same site within the same session4.
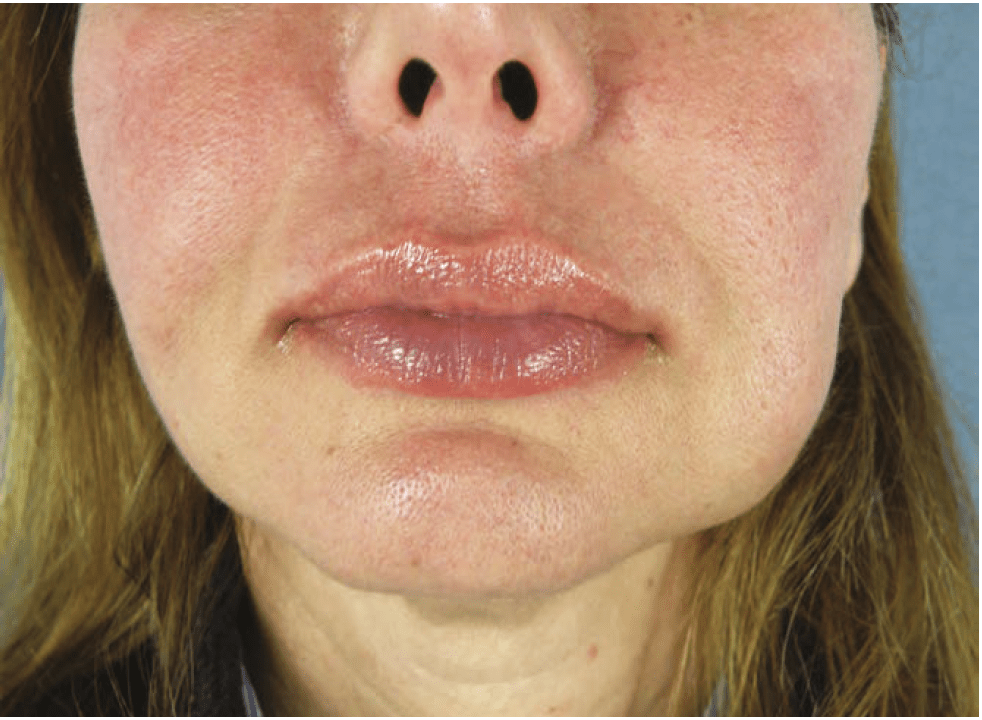
Figure 2 Example of late non-infectious nodules related to a mix of products injected successively
Correct determination of the treatment indications is essential, as is choosing the most appropriate product. Injections should not be attempted if aesthetic improvement of the patient’s face is not guaranteed. The appropriate level should be injected: volumising products should always be injected deeply. The appropriate quantity of product should be injected — too much volume injected at the same time and/or too rapidly can lead to integration problems, but not enough volume may produce an insufficient result.
Resorbable products should be used as a first-line treatment since they are associated with fewer complications than permanent or semi-permanent products. Lastly, the product should not be chosen on the basis of its cost. Products should be chosen according to their rheological characteristics depending on the indication, the level of evidence and clinical safety (ask laboratories for clinical studies). Equally, a limited budget does not justify injecting an inappropriate product in order to only use one syringe; in that case, the treatments should be prioritized.
Nowadays, all fillers should bear an EC mark and ISO security norms for sterilisation, as well as European and American pharmaceutical regulatory norms, with a residual BDDE <2 ppm* (0.002 mg for 1 ml of HA), proteins <2.5 ppm and endotoxins <0.2 EU/syringe. However, there are differences between products, such as rheology, technique and rate of reticulation, particle size, immunogenicity, and hydrophilicity. These properties, which are more or less well known, affect the behaviour of the product in the skin, and therefore, the final result as well as the potential occurrence of unwanted effects.
Before injection:
- The treatment should be carried out in a sterile room dedicated to that purpose
- All make-up should be removed from the face, and antiseptic (chlorhexidine) should be applied
- Hair should be kept away from the operating field, using a hairband or hat
- The doctor should remove his or her watch, rings and bracelets, carry out an antiseptic hand-wash (or a simple hand-wash followed by an application of hydro-alcoholic solution), and put on disposable gloves.
During the injection:
- Avoid touching the needle and cannula
- Change the needle frequently, both to maintain aseptic conditions and because the needle tip becomes blunt and can cause pain, particularly in the case of injections in contact with the bone or in the perioral area
- Use pre-filled syringes as they are, never mix products and never dilute or add any products (e.g. lidocaine)
- Do not inject more than 2 ml per treatment site during one session and do not inject more than 0.5 ml per injection site
- After injection:
- Apply moderate compression (or ask the patient to do so) over the injection points for several minutes to stop any bleeding
- Apply clean cold packs if a haematoma appears
- Ask the patient not to touch the injected site for several hours, not to wear make-up for the first 12 hours, to use a new, unopened face cream and to avoid excessively massaging the area for several days
- During the first 12 hours post-treatment, it is advised not to carry out intense physical exercise, and cardiac frequency should be maintained below 100 beats per minute
- Excessive heat, saunas, Turkish baths and very cold temperatures should be avoided for 2 weeks.
- No dental treatment or maxillofacial orthodontic treatment that could increase the risk of bacteria should be carried out during the first 12 hours.
Resorbable products should be used as a first-line treatment since they are associated with fewer complications than permanent or semi-permanent products.
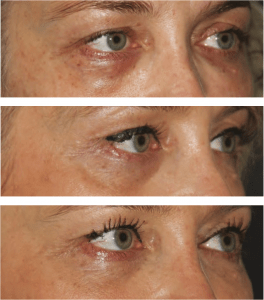
Figure 3 (A) Tyndall effect. (B) After Hyaluronidase treatment. (C) Result after second injection with Volbella®
Treatment
Strict adherence to the protocol described previously considerably limits the incidence of complications from filler injections, but does not totally eliminate them. This section of the article presents the treatments for different complications, in a very practical manner.Complications may be vascular (haematomas, emboli, vascular compressions), inflammatory (immediate or delayed hypersensitivity) or infectious, with particular cases related to malpositioning and/or the use of inappropriate products. Some complications appear early, while others are delayed. Two complications constitute absolute emergencies: anaphylactic shock and ischemia by an embolus or vascular compression.
Early complications: immediate onset (≤ 24 hours)
Ecchymosis and haematoma
Non-serious and always resorbable; however, ecchymosis may be embarrassing for the patient. The patient should, therefore, be informed of the risk, which can never be totally avoided, even with the greatest precaution. It is preferable to plan injections at least 10 days before any important events.
Prevention: stop antiplatelet or anti-coagulant treatment 1 week before injection (if possible), use the finest needles possible or cannula, relax the patient
Treatment: immediate pressure, application of a cool pack, arnica or helichrysum essential oil. A painful haematoma may require the prescription of oral corticosteroids for 4 to 5 days. If it persists, the haematoma may be aspirated.
OedemaCertain sites (lips) may develop post-traumatic oedema immediately after the injection. The oedema is unsightly but disappears in less than a week. The application of a cold pack can accelerate resorption. Prescription of NSAIDs or corticosteroids is rarely necessary. However, oedema may also be the result of an immediate hypersensitive reaction. Prevention: do not inject patients with a history of a reaction to hyaluronic acid (should be picked up in the subjective assessment). Treatment: If the oedema is localised to the injection site, it can be treated with cold packs and oral H1 antihistamines. If the response to the antihistamines is insufficient, corticosteroids may be prescribed. Localised angioedema (lips or eyelids) requires H1 antihistamine injections and rapidly acting corticosteroids (methylprednisolone 1–3 mg/kg or betamethasone 0.1–0.3 mg/kg).
Certain sites (lips) may develop post-traumatic oedema immediately after the injection. The oedema is unsightly but disappears in less than a week.
Vascular complications following accidental intra-arterial injection or arterial compression
Although very rare, this is one of the most serious complications that can occur with injections of hyaluronic acid. Along with anaphylactic shock, it constitutes the only real emergency since:
- Necrosis of the ischaemic cutaneous tissues may occur if the embolus remains in the injection site8–10
- The embolus may travel, although this is rare, by retrograde flow to the ophthalmic artery, branch of the internal carotid artery, via anastomoses11 and can cause blindness or stroke if it continues into the cerebral arteries12–14.
The risk of stroke is around 0.05%15. Only 97 cases have been reported in the literature14. One review reported 75 cases of complete blindness published in the literature, half of which were related to injections of autologous fat and only 20% were attributable to hyaluronic acid16. The majority of these cases occurred in Asia, none have been described in Europe.
If the patient experiences headache or visual disturbances an extension of the embolus to the internal carotid system should be suspected; the patient should, therefore, be sent immediately to the ophthalmic emergency department (retinal ischaemia causes permanent blindness within 90 minutes).
Local ischaemia following arterial occlusion mostly leads to (but not always) intense pain at the time of the injection with immediate blanching of the area, but sometimes it can happen in the following hours, and infiltrated oedema and a livedo pattern appear. The sites that are the most commonly affected (although rarely) are the glabella, the nasolabial folds, and the nose. Complete recovery is usually possible because of the existence of an ‘antidote’ for hyaluronic acid: hyaluronidase, in contrast with fat injections. Hyaluronic acid should, therefore, be the treatment of choice in sites with vascular risk.
Hyaluronidase should be injected in the vitreous or in the territories of the supratrochlear or glabellar arteries, depending on the opinion17–19. Prevention:
- Aspirate before the injection to verify that the needle tip is not in a vessel (not reliable as it is not always positive if the vessel is small)
- The use of a cannula has a lower vascular risk than a needle; however, cases of necrosis have also been described with canulae
- Inject the smallest volume necessary, between 0.1 and 0.3 ml per bolus, displace the needle for each bolus. It is always possible to add product during another session if the result is insufficient
- Inject slowly and monitor the patient’s reactions
- Choose an HA that is adapted to the site: volumising products must only be injected deeply, supraperiosteal, in contact with the bone, and never superficially in the dermis where the vessels lie
- Choose the appropriate injection technique: superficial with progressive filling, using an appropriate HA in the nasolabial folds, for example, or multiple superficial punctures in the glabella
- Have perfect knowledge of facial anatomy and the sites of vascular risk20: Glabella: small vessels and weak collateral circulation increase the risk of necrosis. The proximity of the supratrochlear and supraorbital arteries, as well as branches of the ophthalmic artery, which originate from the internal carotid artery, causes a risk of retrograde embolization to the ophthalmic artery
>> Nose bridge: the dorsal nasal artery is the terminal branch of the ophthalmic artery with little collateral circulation, and thus there is a risk of retrograde embolization
>> Superior portion of the nasolabial fold and alar area where the facial artery (external carotid) becomes superficial: cutaneous necrosis is most common here (although rare)20,21
>> Palpebral nasal fold where the angular artery is superficial
>> Temples: due to the connection between the temporal artery and the supraorbital artery
>> The fleshy part of the lips where the product is injected very close to the labial artery22. The border of the lips is not a risky site.
Treatment:
- Immediately stop injecting if pain occurs or if the site becomes livid
- Urgently review any patient who complains of pain, swelling, and blanching of the injected site in the ensuing hours
- Apply hot compresses and massage in an attempt to free the vessel and avoid necrosis
- Inject hyaluronidase with small boli (dilution 1500UI/ 5cc NaCl serum) in and around the site and massage gently (too vigorously may push the hyaluronidase away from the site). As soon as it is injected, hyaluronidase diffuses widely in the subcutaneous tissues and dissolves intravascular hyaluronic acid23.
- Other treatments as vaso-dilators, aspirin 80 mg, or low molecular weight heparin, 175 UI/kg/day, for a maximum 10 days, are of little use and transcutaneous nitrated derivatives (vasodilators)24 and hyperbaric oxygen is controversial24,25.
- Paracetamol 4g/day in case of pain
- Use of transcutaneous nitrated derivatives (vasodilators)24 and hyperbaric oxygen is contraversial24, 25. Corticosteroids and ibuprofen are contraindicated.
- Review the patient daily and take pictures
- In the case of eye symptoms, send the patient immediately to the ophthalmic emergency department or to a dedicated specialist physician and recommend a peribulbar or retrobulbar injection of hyaluronidase17,18 (1.000 U).
Early complications (first days or weeks)
Reactivation of herpetic infection
Herpetic vesicles are usually quite typical at the start of an eruption, but at the stage of crusts, or in the case of hyper-infection, they can be mistaken for an ischaemic problem.
Prevention: before injection, question the patient regarding any history of herpes around the face or eye, if necessary, prescribe a preventative treatment (acyclovir) a few days before the session.
Treatment: local plus systemic aciclovir, local antiseptic.
Bacterial infections
Verify that there is a true infection (complete blood count and sample plus antibiogram), and not an aseptic phenomenon. In the presence of a foreign body, such as a filler, the number of bacteria required to cause a clinical infection is considerably reduced6.
Prevention: adhere strictly to recommendations for cleanliness and asepsis; do not inject patients who are immunosuppressed; use fine needles and limit the number of entry points.
Treatment: antibiotics (if positive infection): doxycycline, and if no improvement after 3 weeks: levofloxacin 500 mg once per day and clarithromycin 250–500 mg twice per day7. Take care if injecting hyaluronidase in the presence of infection as it may extend the infection. It is preferable to wait until the infection is controlled by antibiotics6.
Nodules
A nodule that appears early can result from many situations. The patient should be examined to determine if the nodule is fluctuating and/or inflammatory.
A fluctuating inflammatory nodule requires sampling and cultures to determine if it is infectious or not — an inflammatory, non-fluctuating nodule requires a biopsy for histological diagnosis.
- Non-fluctuating non-inflammatory nodule: it may be an encapsulated haematoma, usually painful, or a mass of malpositioned or an excessive dose of hyaluronic acid.Treatment: aspiration of the haematoma; injection of hyaluronidase if there is an accumulation of the product
- Inflammatory nodule: rarely caused by infection, either fluctuating or non-fluctuating equals non-infectious collection caused by alteration of the product, such as a dangerous mix of products, as PDO and HA or monophasic and biphasic HA. Treatment: hyaluronidase if non-fluctuating or puncture-aspiration-drainage and cultures if fluctuating. Corticosteroids can be helpful. Empirical antibiotics (clarithromycin plus quinolone for 2 to 4 weeks) if nothing works.
Visibility of the product due to malpositioning, Tyndall effect, or malar oedema
Prevention: a sound knowledge of anatomy is essential, use a product that is adapted to the treatment site and the depth of the injection; inject small quantities, more can be injected during subsequent sessions.
Treatment: repositioning massage can be effective during the first 48 hours after injection if the quantity of product is small and the malpositioning is relative. Due to the risk of an allergic reaction, hyaluronidase should not be used indiscriminately to correct poor injection techniques, it should be reserved for the treatment of true side-effects.
Malar oedema8
Oedema is caused either by injecting too superficially in front of the orbital-malar septum, or an excess of product. The result is a bag-like appearance.
Prevention: All fillers injected in the malar septum should be placed deeply, subperiosteally and especially, out-with any malar bags. The smallest quantity possible should be used to avoid malar oedema as well as superficial nodules and the visibility of the filler.
Treatment: hyaluronidase.
Tyndall effect
A discolouration (bluish) caused by a too superficial injection of hyaluronic acid (with the absorption of the short lengths of blue waves), often visible under the eyes where the skin is very fine, and there is little subcutaneous tissue.
Prevention28:
- Inject deep (under the orbicularis muscle), avoid superficial injection
- Inject a small quantity to avoid overcorrection
- Inject slow, gently with less force of extrusion to avoid spreading.
Also, it’s better to use a hyaluronic acid gel with minimal water absorption and in small concentrations.
Treatment: hyaluronidase.
Delayed complications (after 4 weeks)
Early oedema or nodules that persist for more than 3 weeks or that appear late
This would suggest a delayed hypersensitivity, a granuloma, infection by atypical mycobacteria, systemic disease or a biofilm (the concept of which is currently debated).
Treatment: corticosteroids
Assessment: biopsy, anti-nuclear antibody screening, liver function tests, serology, kidney function tests, and inflammatory assessment.
Delayed hypersensitivity
Characterised by indurated oedema and erythema that appears between 24 hours and several weeks after the injection. It is mediated by T-lymphocytes and thus does not respond to antihistamines8.
Biofilm
Is an aggregate of microorganisms that form a net, imprisoning leucocytes. It is a slow, dormant infection that is highly resistant to the usual antibiotics, and cultures are often negative. This concept is currently becoming abandoned.
 Granuloma
Granuloma
Is diagnosed by histological analysis. It may present as one or several nodules or infiltrated plates, which can be more or less inflammatory and more or less painful. In this case, it is usually a non-allergic, foreign body granuloma, dominated by foreign body giant cells, which also contain histiocytes, lymphocytes, and other inflammatory cells31. The function of the reaction is to isolate and prevent the migration of the foreign body that cannot be rapidly eliminated by phagocytosis or dissolved by enzymes. The organism, therefore, proceeds to sequestrate the injected material with a capsule of monocytes and macrophages. The contents resist the degradation, and the activated macrophages secrete pro-inflammatory cytokines8. Granulomas nearly always occur when the filler has been injected in a site previously treated by non-resorbable products. A latent infection may favour the formation of granulomas6.
In practice, a biopsy should be carried out to confirm the diagnosis. Late-occurring inflammatory nodules should be treated with antibiotics (doxycycline or a combination of clarithromycin 500 mg/day and levofloxacin 500 mg/ day for 2 to 4 weeks), injection of hyaluronidase, and possibly intralesional corticosteroids once the infection has been controlled. As a last resort, the granuloma may be excised surgically6.
Multiple nodules suggest the presence of systemic disease and connective tissue disease should be assessed.
Declaration of interest The authors declare that Allergan provided support to the preparation of the manuscript (medical writing and translation)
Figures 1–3 © Lakhdar Belhaouari
References:
- J Cohen MD. Understanding, Avoiding, and Managing Dermal filler complications. Dermatol Surg 2008;34:S92–S9
- Bailey SH, Cohen JL, Kenkel JM. Etiology, Prevention, and Treatment of Dermal Filler Complications Aesthetic Surgery Journal 2011 31: 110
- De Lorenzi Complications review Part I – Aesthetic Surgery Journal 2013 – 33:561
- De Boulle K, Heydenrych I. On behalf of the Consensus Group. Patient factors influencing dermal filler complications: prevention, assessment, and treatment. Clin Cosmet Investig Dermatol. 2015;8:205-14
- Descamps V, Landry J, Francès C, Marinho E, Ratziu V, Chosidow O. Filler Injections as Possible Target for Systemic Sarcoidosis in Patients treated with Interferon for Chronic Hepatitis C: Two Cases. Dermatology 2008;217:81–84
- Wagner RD, Fakhro A, Cox JA, Izaddoost SA. Etiology, Prevention, and Management of Infectious Complications of Dermal Fillers. Semin Plast Surg. 2016;30(2):83-6
- Signorini M, Liew S, Sundaram H, De Boulle KL, Goodman GJ, Monheit G, Wu Y, Trindade de Almeida AR, Swift A, Vieira Braz A; Global Aesthetics Consensus Group. Global Aesthetics Consensus: Avoidance and Management of Complications from Hyaluronic Acid Fillers-Evidence- and Opinion-Based Review and Consensus Recommendations. Plast Reconstr Surg. 2016;137(6):961e-71e
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295-316
- Beleznay K, Humphrey S, Carruthers JD, Carruthers A ; Vascular compromise from soft tissue augmentation. Experience from 12 cases and recommendations for optimal outcomes. J Clin Aesth Dermatol 2014; 7: 37-43
- Inoue K, Sato K, Matsumoto D, Gonda K,Yoshimura K: arterial embolisation and skin necrosis of the nasal ala after dermal filler Plast Reconst Surg 2008;121(3):127-128
- Kwon DY, Park MH, Koh SB, Dhong ES, Baek SH, Ryu HJ, Park KW. Multiple arterial embolism after illicit intranasal injection of collagenous material. Dermatol Surg 2010;36:1196–1199
- Lazzeri D, Agostini T, Figus M, Nardi M, Pantaloni M, Lazzeri S. Blindness following cosmetic injections of the face. Plast Reconstr Surg. 2012;129(4):995-1012.
- Park SW, Woo SJ, Park KH, Huh JW, Jung C, Kwon OK. Iatrogenic retinal artery occlusion caused by cosmetic facial filler injections. Am J Ophthalmol. 2012;154(4):653-662.
- Beleznay K, Carruthers JD, Humphrey S, Jones D. Avoiding and Treating Blindness From Fillers: A Review of the World Literature. Dermatol Surg. 2015;41(10):1097-117.
- Beleznay K, Humphrey S, Carruthers JD, Carruthers A. Vascular compromise from soft tissue augmentation: experience with 12 cases and recommendations for optimal outcomes.J Clin Aesthet Dermatol. 2014;7(9):37-43
- Li X, Du L, Lu JJ. A Novel Hypothesis of Visual Loss Secondary to Cosmetic Facial Filler Injection. Ann Plast Surg. 2015 Sep;75(3):258-60.
- Goodman GJ. A rethink on hyaluronidase injection, intraarterial injection, and blindness: is there another option for treatment of retinal artery embolism caused by intraarterial injection of hyaluronic acid? Dermatol Surg 2016;42:547–549
- Fagien S. Commentary on a Rethink on Hyaluronidase Injection, Intra-arterial Injection and Blindness. Dermatologic Surgery. 2016;42(4):549-52
- Steinsapir KD. Treating Filler Related Visual Loss. Dermatol Surg. 2016;42(4):552-4
- Grunebaum LD, Bogdan Allemann I, Dayan S, Mandy S, Baumann L. The Risk of alar necrosis associated with dermal filler injection. Dermatol Surg. 2009;35 Suppl 2:1635-40.
- Kang MS, Park ES, Shin HS, Jung SG, Kim YB, Kim DW. Skin necrosis of the nasal ala after injection of dermal fillers. Dermatol Surg. 2011 Mar;37(3):375-80.
- Vent J, Lefarth F, Massing T, Angerstein W. Do you know where your fillers go? An ultrastructural investigation of the lips. Clin Cosmet Investig Dermatol. 2014;7:191-9.
- De Lorenzi C. Complications of Injectable Fillers, Part 2: Vascular Complications. Aesthet Surg J. 2014;34(4):584-600
- Hwang CJ, Morgan PV, Pimentel A, Sayre JW, Goldberg RA, Duckwiler G. Rethinking the Role of Nitroglycerin Ointment in Ischemic Vascular Filler Complications: An Animal Model With ICG Imaging. Ophthal Plast Reconstr Surg. 2016;32(2):118-22.
- Darling MD, Peterson JD, Fabi SG. Impending necrosis after injection of hyaluronic acid and calcium hydroxylapatite fillers: report of 2 cases treated with hyperbaric oxygen therapy. Dermatol Surg. 2014;40(9):1049-52
- Hirsch RJ, Narurkar V, Carruthers J. Management of injected hyaluronic acid induced Tyndall effects. Lasers Surg Med. 2006;38(3):202-4.
- Rootman DB, Lin JL, Goldberg R. Does the Tyndall effect describe the blue hue periodically observed in subdermal hyaluronic acid gel placement? Ophthal Plast Reconstr Surg. 2014;30(6):524-7.
- Belhaouari L, Gassia V. L’art de la toxine botulique et des techniques combinées en Esthétique; éditions Arnette Wolters Kluver France; 2013
- Narins, R Coleman W. Treatement options for nodules and other filler complications. Derm Surg 2009; 35 Suppl 2:1667-71.
- Alijotas-Reig J. Inflammatory, immune-mediated adverse reactions related to soft tissue dermal fillers, www.elsevier.com/locate/semarthrit Seminars in Arthritis and Rheumatism.
- Fernandes S & Soares de Almeida L. Granulome à corps étrangers. Thérapeutique Dermatologique. 15 mai 2013. http://www.therapeutique-dermatologique. org/spip.php?article1136



 Granuloma
Granuloma