A research team from Everon Biosciences, Inc. and Roswell Park Cancer Institute has identified a specific subpopulation of immune cells accumulating in old mice that are likely contributors to aging and age-related diseases. Previously, this function had been solely attributed to senescent cells, which have been declared to be the major target for anti-aging therapies.
This discovery leads to a major revision of the previous paradigm by broadening focus of anti-aging drug discovery and development to include a new likely cellular source of age-associated chronic systemic sterile inflammation. The study was published online today ahead of print in the scientific journal Aging.
The newly described age-associated cells represent a specific subtype of macrophages, one of the major components of innate immune system normally involved in the removal of solid cellular waste. This subtype, named by the authors “senescence-associated macrophages” or SAMs, share some biomarkers of senescent cells by expressing p16(Ink4a) gene and an enzyme beta-galactosidase. For this reason, this study suggests accumulation of SAMs in tissues of old mice may have been erroneously interpreted as accumulation of senescent cells.
“This finding does not contradict the current dominant view on aging, which is viewed as a consequence of increased inflammation due to cells secreting proinflammatory factors. Rather, we are suggesting a switch in focus from senescent cells to SAMs” said Andrei Gudkov, Ph.D., DSci, Chief Scientific Officer of Everon Biosciences and Chair of Cell Stress Biology at the Roswell Park Cancer Institute.
Dr. Gudkov continued, “Recognizing SAMs as a cellular target of aging allowed us to timely redirect our effort from senolytic to ‘SAMolytic’ drugs and to accelerate the development of novel anti-aging therapeutics.”
Previous studies demonstrated signs of rejuvenation of mice following eradication of p16(Ink4a) cells. The current study finds the p16(Ink4a) cell population largely consists of SAMs, thus providing powerful argument in favor of significant contribution of SAMs to aging.
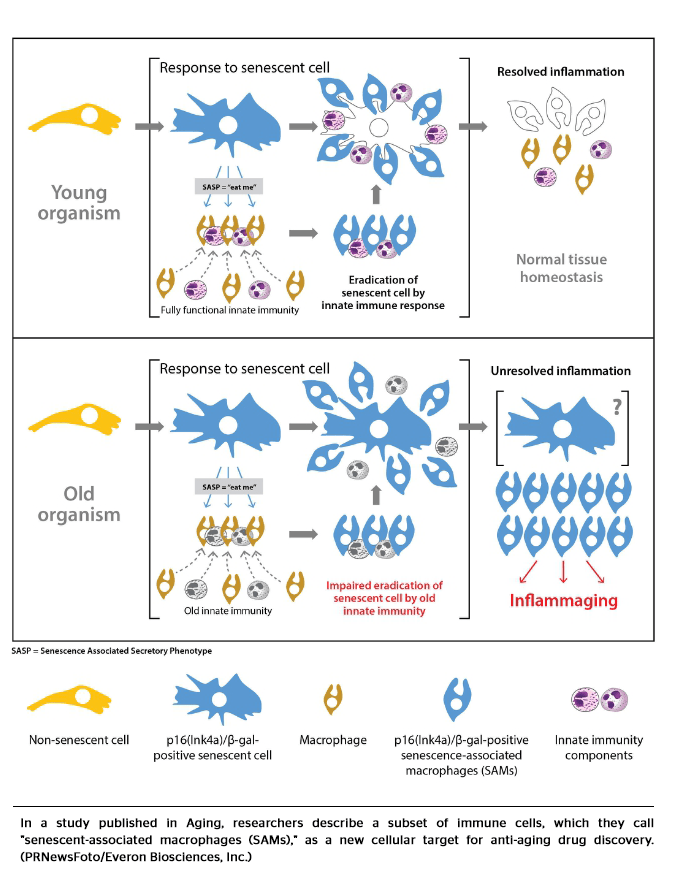
The study, “Aging in mice is associated with p16(Ink4a)- and beta-galactosidase-positive macrophage accumulation that can be induced in young mice by secreted factors of senescent cells,” will be published in print in the July issue of Aging.
The article can be found online here: http://www.aging-us.com/article/XFECL8coa6th4i87b.
In addition, Dr. Gudkov has presented these new data today at the Research Workshop of the Israel Science Foundation 2016 called Cellular Senescence: From Molecular Mechanisms to Therapeutic Opportunities, which attracted top internationally recognized experts in senescence and aging. The conference is taking place at the Weizmann Institute of Science in Rehovot, Israel.
About Everon Biosciences
Everon Biosciences, Inc. is a private biotechnology company focused on developing novel anti-aging therapeutics. Aging is a systemic process of progressive dysfunction of tissues and organs associated with chronic inflammation, changes in tissue composition and the quality of cells within the tissues. Everon is a leader in understanding cellular mechanisms of aging and translating this knowledge into development of anti-aging therapies. Everon Biosciences, Inc. is headquartered in Buffalo, New York.
For more information, please visit www.everonbio.com.





