Adele Sparavigna, MD discusses the results of her study using
two different injection techniques, resulting in a reduction
in the signs and symptoms of cellulite

email: [email protected]
Panniculopathy edematous-fibro-sclerotic (PEFS), more commonly referred to as cellulite, affects at least 90% of the post-pubertal female population; and the male population is not entirely exempt from it either. This condition is based on histological, biochemical and ultrastructural observations. Furthermore, comorbidities such as obesity and the venous pathology of the lower limbs, may also play a role. Certainly, the histological aspects that clearly represent the complexity of the pathogenesis of this disease are characterised by three essential events: alterations of the adipose tissue, hyper-polymerisation of the connective tissue matrix, and profound alterations of the microcirculation.
Female sex hormones play a significant role in the development of cellulite, which explains why this pathology occurs almost exclusively in the female gender.
The main pathogenic mechanism of cellulite is the altered capillary-venular permeability, which results in a slowing of the speed and volume of blood flow (vasomotion) at the local micro-circulatory level.
The particular conformation of the micro-vascular-tissue unit justifies the fact that the defects to the microcirculation in the area are often associated with alterations in the blood flow that extend to the skin and regional muscles, causing clinical effects such as hyperkeratosis (orange peel skin), tenderness and, in the more advanced stages, profound subversion of the loco-regional aesthetic (mattress skin). The presence of estrogen receptors in endothelial cells and smooth muscle explains the functional differences of female microcirculation, especially in relation to vascular tone and permeability and, therefore, the greater susceptibility of the female sex to cellulite.1
Recent studies inform us that, at the level of cellulite, among the functional alterations encountered by adipocytes, there is an increase in the release of phlogistic cytokines. Therefore, adiposity involves the activation, at a localised level, of inflammatory biochemical-cellular mechanisms.
These findings are absolutely consistent with the estrogenic activity involved in the development of cellulite, which has been widely described and has been further ascertained with the observation of female tissue undergoing hormonal therapies and monitored by imaging techniques, such as ultrasound and computerised axial tomography.2
Once the process is triggered, over time it develops due to the inflammatory processes described above. On the one hand, the neo-synthesis of collagen leads to the formation of fibrous branches; on the other, a reduction of the compactness of the tissues around the fibrous branches is also witnessed. This increase in skin laxity seems in some way similar to the manifestations of skin ageing, also characterised by a degradation of the dermo-epidermal fibrous matrix.
Injection Treatment
Injection therapy has always been considered one of the most viable solutions for the treatment of cellulite. Of course, it is very important to identify the most suitable product for each case, having clearly in mind the mechanism of action and the expected results, but also the most suitable injection technique to be used. Treatment of the extracellular matrix (ECM) is certainly the primary goal.
As we have seen, the alterations at the ECM level play a fundamental role in the genesis of cellulite.
It has been demonstrated that on histological samples of animal models it was possible to evaluate that physiological elastinogenesis and collagenogenesis, induced in a specific dermal district, have been shown to be effective in reducing skin laxity, mainly correlated to the manifestations of cellulite.
The product ‘Sunekos® Cell’ (SC), proposed for the study, contains a patented mixture of six structural amino acids fundamentally and specially formulated for an optimal neo-synthesis of collagen and elastin in the dermis. It has already been demonstrated that a functional cluster of amino acids is suitable for promoting in-loco the synthesis of collagen and elastin.3,4
In expert hands local injection of this formulation can be a powerful tool because chemotaxis induced by the presence in the dermis of specific amino acids for the production of collagen and elastin is known to be the strongest force in nature that drives life: living things are attracted to food. In fact, we are sending a very strong biological message to the fibroblasts. Feeding the fibroblasts with the right food (the right amino acids) also results in restoring a ‘normal’ structure of the dermal architecture.
Additionally, a naturally low molecular weight hyaluronic acid (HA) (200,000 Daltons) is also synergistically present in the Sunekos® Cell formula, which exerts a targeted stimulating action on the fibroblasts even through the binding of the specific receptors for the HA fragments.
A bicarbonate/carbonate buffer has been added to the standard formula of Sunekos® to Sunekos® Cell in order to achieve a pH of 8.2 for specific use in panniculosis. The bicarbonate and carbonate solution buffers local acidosis due to the state of latent inflammation that characterises both the early and late stages of the condition.5
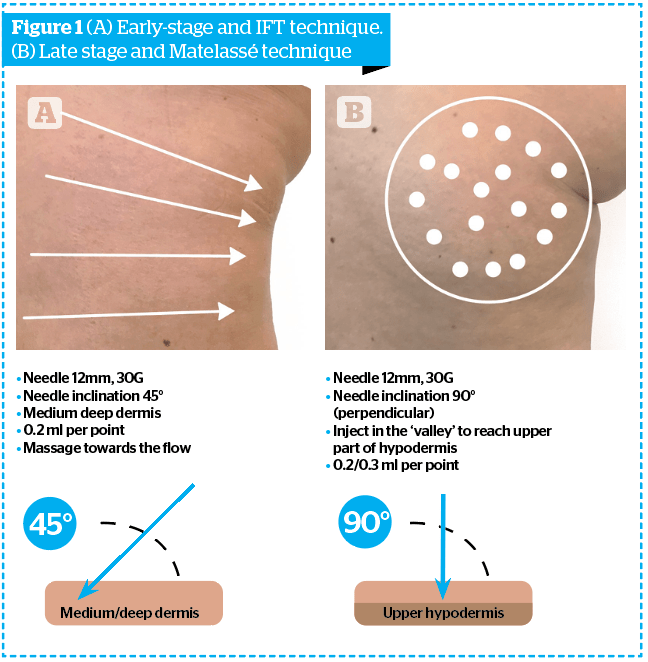
The disruption of the extracellular matrix of the dermis and hypodermis can be treated effectively, depending on the level and injection technique used. In the early stages, it is important to treat the deep dermis, both for the action on the interstitial fluids and the fat cells of the dermis, which we have seen are able to trigger the pathogenetic mechanism. This mechanism promotes fibroblasts capable of migrating and of altering the ECM of the hypodermis, in order to restore the physiological interstitial fluid movement towards the lymphatic circulation. In the late stage, the aim will be to rearrange the structural and architectural disorder of the fibrous septa of the hypodermis. Injecting with Sunekos® Cell should therefore be exercised at a deeper level, in the fat adipose tissue. To summarise, when the treatment is for early-stage cellulite and mainly involves the dermis, the injection technique will be at the level of the deep dermis; taking into account the product, thanks to its low viscosity characteristics, will spread optimally throughout the surrounding area. Alternatively, if we are treating late-stage cellulite, the treatment will be at the level of the more superficial hypodermis, so as to exert a non-invasive action. Contrary to what is often stated by other authors, it is not necessary to violently disrupt fibrous branches, which is commonly done with large-calibre cannulas and often even with a ‘bayonet’ cutting tip. The traumatic action of breaking the fibrous branches, in addition to systematically provoking at this level the formation of large bruises and hematomas (which often remain in unsightly hemosiderin hyperpigmentation) greatly distances the objective of ‘restitutio ad integrum’. The preferred technique is to reach the hypodermis with a smaller gauge needle and inject an adequate amount of the SC product, which will quickly spread alone in a large surrounding area. In more detail, in the initial phase it will be possible to use the method we already know as the Interstitial Fluid Technique (IFT), that is, making sure that the injecting treatment is able to push the movement of the interstitial fluids towards the local lymph node stations, achieving improved lymphatic drainage due to the massaging of the injected product.6 The injection is then followed by a light manual massage in the same direction. Since, in the early stages of panniculosis, the problem of stagnation of interstitial fluids, together with alterations of the micro-vascular-tissue exchange, and the acidosis typical of the state of latent inflammation of the disease is responsible for disruption to the ECM. Therefore, it is important to counteract the problem using the specific Sunekos® Cell formula together with a targeted technique. For IFT, it is advisable to use a 27–30 G needle, 12 mm long, with an inclination of 45 ° with respect to the skin surface, made to penetrate for half-length into the skin. We will then inject 0.3–0.5 cc per point, with the points arranged along radial lines converging from the lateral surface to the medial surface of the thigh, in the direction of the inguinal lymph nodes anteriorly and inguinal-crural posteriorly. The number of points (2 cm apart) and lines, will obviously vary depending on the extent of oedema and the orange peel skin (Figure 1A).
As for the more advanced stages, in order to obtain a rearrangement of the fibrous septae in the least traumatic way possible, the same type of needle will be used, 27–30G, 12 mm in length, but this time inserted perpendicularly to the skin surface penetrating for the entire length and injecting 0.3–0.5 cc of product per point (Matelassé technique). Each point will be on lines drawn in the deepest depressions of the skin surface (mattress skin) at a distance of about 1.5 cm from each other (Figure 1B).
To draw the areas to be treated, the patient must be examined in an orthostatic position, preferably in a well-lit environment from above and slightly to the side, in order to accentuate the imperfections to be treated, tracing the lines most suitable for the case in question.
Study objective
The aim of this study was to evaluate by clinical, morphometric, and non-invasive instrumental evaluations the efficacy of an injective treatment on signs and symptoms of cellulite.7 Sunekos® Cell is a CE Class III medical device composed of 7 ml of linear hyaluronic acid and specific amino acids to be mixed with a 3 ml solution of carbonates and bicarbonates. The trial was performed on healthy female volunteers with moderate edematofibrosclerotic panniculitis (EFP) on the thighs. Sunekos® Cell was injected by needle (27-30G length 13mm), and the needle was inserted into the medium-deep dermis (45°) at the level of the trochanteric region — following the IFT pattern in case of early-stage cellulite (orange peel appearance, following a fan-shaped pattern across the thigh area converging towards inguinal lymph-nodes); and perpendicularly to the skin (90°) in the most deep furrows in case of late-stage cellulite (mattress appearance). The distance between injection points was 1.5 cm.
An additional aim of this study was to evaluate the treatment tolerance.
This study had obtained the approval of an Independent Ethic Committee on 16 January 2020.
Study treatment
The study was conducted on 25 female adult subjects, age range: 33–60 (mean age: 48 years.), whose written consent had been obtained. The patients were divided into two groups during the basal visit:
- Group A (early stage): patients with grade 1–2 as per visual aspect grade: 11 patients
- Group B (late stage): patients with grade 3–4 as per visual aspect grade: 14 patients.
- The study treatment foresaw two injection sessions at an interval of 10 days using the IFT (for Group A) and the Matelassé technique (for Group B) depending on the cellulite stage.
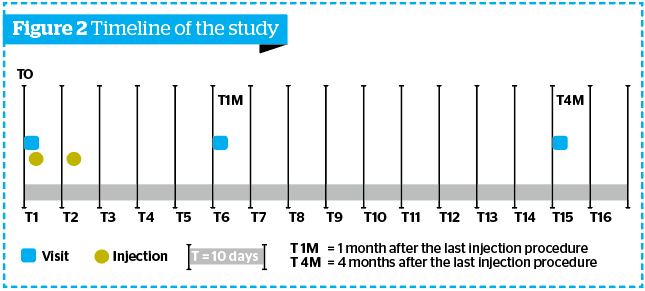
- Four visits were performed during the study (Figure 2):
- T0: basal assessments, followed by the first injection procedure
- T2i: preliminary assessment and second injection procedure
- T1M (1 month after the last injection procedure: evaluations foreseen by the study protocol (Follow-up phase)
- T4M (4 months after the last injection procedure).
Data processing and statistical analysis were performed as follows: Friedman test followed, in case of a statistically significant result, by a Holm-Sidak Adjusted test.
The inclusion and exclusion criteria will be available on the full study that will be published soon.
All evaluations were carried out mono-laterally on the right or left thigh (third superior), according to a subjects’ randomisation list defined by the investigator before the subjects’ inclusion. At T0, T1M, and T4M clinical, morphometric and instrumental assessment were performed before the injection procedure.
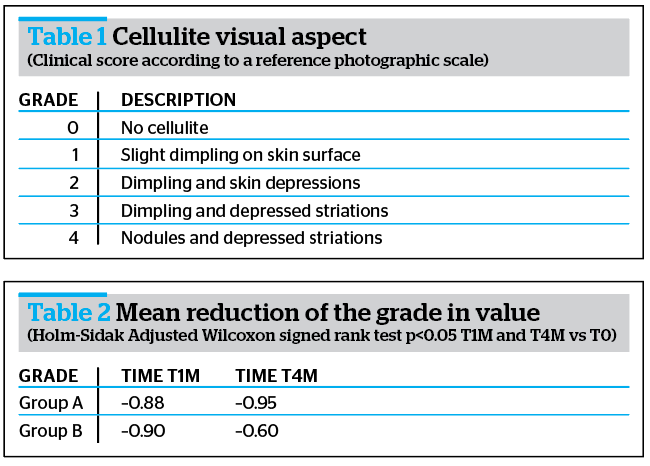
Clinical evaluations were performed according to the visual score described in Table 1.
Photographic documentation
At T0, T1M, and T4M, 2D pictures of the treated areas (left or right side randomly) for all included volunteers were taken.
Circumferences measurement
All the measurements were performed in standard conditions at the level of the thigh (middle thigh and under gluteus), thanks to a specific electro-optical system able to fix the volunteer’s position. To reduce the intra-individual variability, circumference measurements were performed three times for each level.
Ultrasonographic evaluation
Ultrasonographic measurement of adipose panniculum thickness (mm) was performed at the level of the third superior (external side), through the instrument Body Metrix™ BX 2000 (Genex). The BX 2000 generates an ultrasound signal (5 MHz) that propagates through tissue and then records the reflected signal.

Results
Clinical assessment
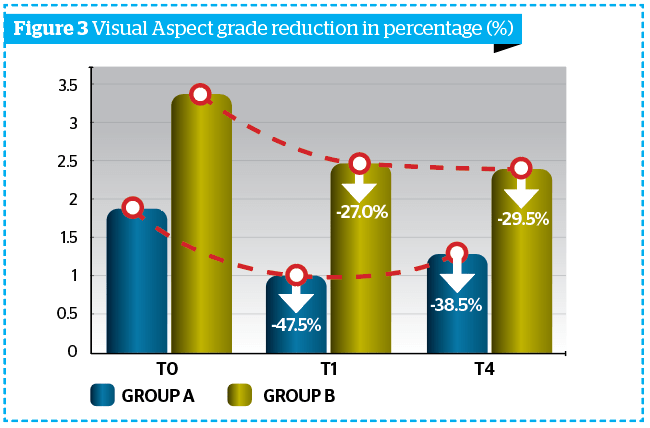
Obtained results highlighted a reduction of cellulite visual aspect of at least 1 degree in 92% of all subjects (Group A + Group B) vs T0, according to DERMING reference photographic scales and a mean reduction of the clinical degree of cellulite (Table 2 and Figure 3).
Morphometric evaluation
All circumferences measurements were performed in standard conditions thanks to a specific electro-optical system able to fix the volunteer’s position.
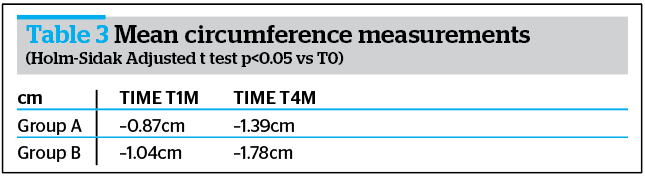
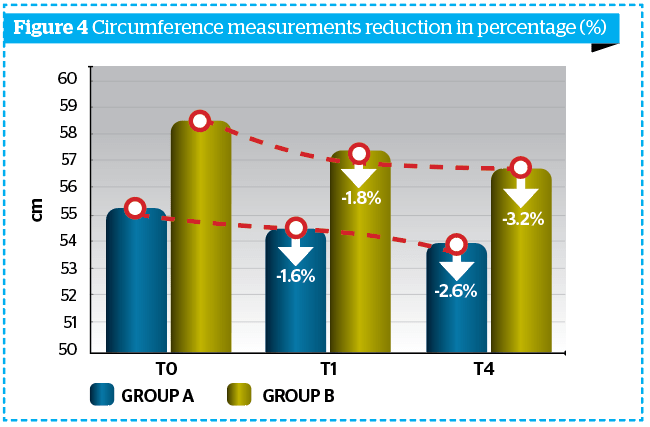
Morphometric evaluations underlined the activity of the treatment; in fact, already at T1M and at T4M the mean value of the thigh and circumferences were already reduced, when compared to baseline in both groups (Table 3 and Figure 4).
Ultrasound assessment
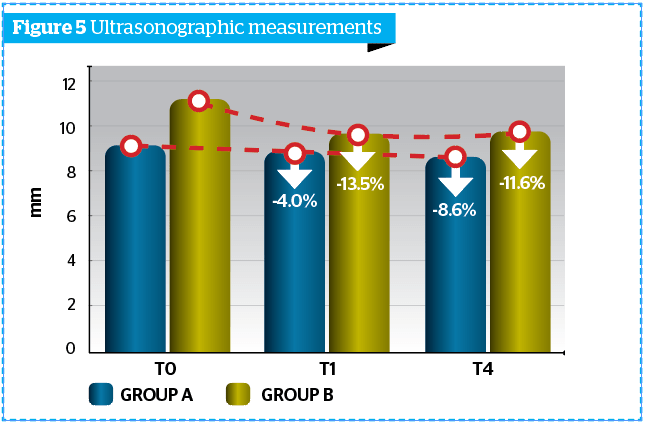
Ultrasonographic measurement of adipose panniculus was performed through the instrument Body Metrix™ BX 2000 (Genex). Obtained results were more marked at T1M and at T4M clinically and statistically significant reduction of the panniculus thickness (mm) vs T0, index of a lipo-reducing efficacy of the tested product (Figure 5).
Tolerance evaluation
No adverse event/reaction (nor expected as pain, discomfort, bruises neither related to the study product) occurred during the trial. The very high tolerance (100%) was confirmed by the investigator at the end of the treatment period.
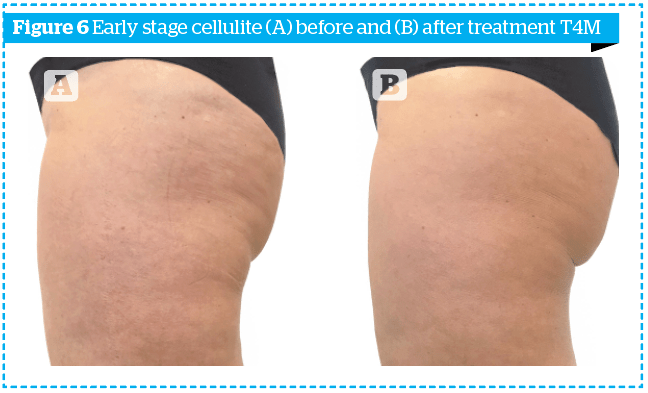
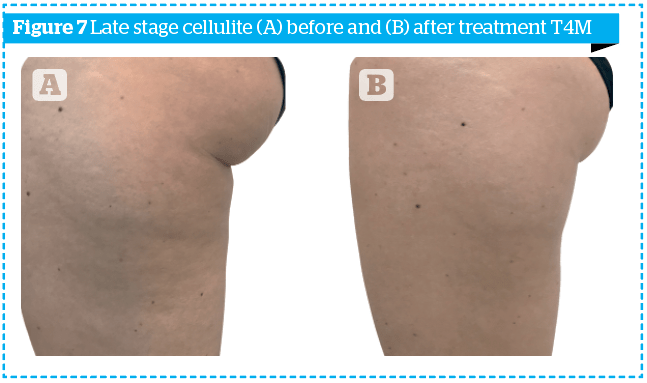
In order to highlight the results obtained not only numerically, the photographs of two patients, one of the early group and one of the late group, at time T0 and time T4M, are shown in Figures 6–7.
Conclusion
Sunekos® Cell is able to counteract the dermal damage, which seems to be the most important triggering of the condition and acidosis at the local level. The injection techniques have to be planned, taking into account the phase of the disease in:
- early-stage cellulite the Interstitial Fluid Technique is the most suitable (Figure 1A)
- late-stage cellulite the Matelassé Technique has demonstrated to be efficacious (Figure 1B).
The first findings of this study demonstrate efficacy, safety and the effectiveness of the injection technique dependent on which stage of cellulite identified. In addition, the experience gained from this initial part of the study has provided evidence to determine the exact quantities of the product to be used and the number of sessions appropriate to each individual type of patient. The final data will be published shortly in the full study.
Declaration of interest Derming received a grant from Professional Dietetics for conducting this research
Figures 1–7 © Dr Adele Sparavigna
Tables 1–3 © Dr Adele Sparavigna
References
- Sbarbati A. L’ultrastruttura della cellulite” in “Cellulite. L’ Alzheimer della pelle” edito da G.Amuso et al. , Libreria Cortina Milano, 2018, p.99-104
- Kinney BM, et al. Histological examination of skin tissue in the porcine animal model after simultaneous and consecutive application of monopolar radiofrequency and targeted pressure energy. J. Cosmetic Dermatology 2020, 19: 93-101
- De Servi B., Orlandini A., Caviola E. and Meloni M. Amino acids and hyaluronic acid mixtures differentially regulate extra cellular matrix genes in cultured human fibroblast. J Biol. Regul. & Homeost. Agents, Vol. 32, no. 3, 517-527, 2018
- Dioguardi FS. Nutrition and skin. Collagen integrity: a dominant role for amino acids. Clinics in Dermatology 2008, 26:636-640
- Heydecker FC, Albergati FG. Valutazione dell’attività di Basentabs nella PEFS agli stadi intermedi in associazione a Lymidiarale Veno N. Medicina Funzionale, 2007, 2:2-7
- Sparavigna A, Guglielmini G, Togni S, Cristoni A, Maramaldi G. Evaluation of anti-cellulite efficacy: a topical cosmetic treatment for cellulite blemishes – a multifunctional formulation. Int J Cosmet Sci, 2011, 33(6):519-26.
- Sparavigna A., Orlandini A.. Efficacy and Tolerance of an Injectable Medical Device Containing Hyaluronic Acid and Amino acids: A Monocentric Six-Month Open-Label Evaluation J Clin Trials volume 7, issue 4, 1-6, 2017





