Sources of oestrogen exposure
Evidence is being accumulated to make it clear that oestrogen exposure from both exogenous and endogenous sources is an important factor in developing hormone-sensitive cancers. Environmental oestrogens can induce, overload and potentially modify some of the SNPs involved in oestrogen detoxification. This can possibly lead to the production of more harmful oestrogen metabolites and also have the ability to mimic harmful oestrogens in the body22, 23. These environmental oestrogens are responsible for breast cancer being labelled an environmental disease. These are phthalates from cosmetics, polychlorinated biphenyls, dioxins, polycyclic aromatic hydrocarbons, perchloroethylene, and phenols, but also oral contraception, cimetidine, hormones in animal products, and conventional HRT for example.
Endogenous oestrogens such as estradiol, estrone, estriol, hydroxylated and methoxylated oestrogen metabolites, as well as oestrogens from enterohepatic recirculation, and their ratios could also be influenced by obesity, alcohol consumption, insulin resistance, and ageing. Alcohol consumption in particular, when combined with HRT, synergistically increases the risk of breast cancer24, 25. Ageing and menopause, a high body mass index (BMI), and the presence of inflammatory cytokines TNF-α, IL-1 and IL-6, increase the aromatase activity that converts androgens into oestrogens, increasing a local source of oestrogens that facilitates the growth of hormone responsive tumour cells. Oestrogen dominance as a result of hormonal imbalances between oestrogen, progesterone and testosterone can further complicate the oestrogen burden.
Nutritional factors and dietary oestrogens can influence receptor expression and modify SNP activity, affecting the detoxification pathways of oestrogen. This is a foundation of nutrigenetics that is used in patients with an unfavourable oestrogen metabolism genetically determined to modulate and improve detoxification.
Genetic testing for increased HRT safety and personalised prevention
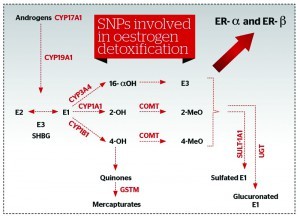
Testing low penetrance genes involved in oestrogen metabolism are useful diagnostic tools to identify a patient’s individual metabolic pathways, and to calculate the possible risk for breast cancer, as well as allowing physicians to tailor a personalised approach to HRT. None of these polymorphisms would significantly increase the risk of breast cancer on their own, but in the presence of prolonged oestrogen exposure or other relevant dietary and lifestyle risk factors, the risk for breast cancer is significantly increased26, 27. Furthermore, a combination of certain polymorphisms can increase the incidence of breast cancer (e.g. COMT in combination with GSTM1) (Figure 5).
Laboratoires Réunis (Luxemburg) provides a comprehensive genetic test (Femgen) that can help to obtain a clear picture of SNPs present in a particular case, as well as their predictive activity. This test should be used as an additional tool to blood and urine samples (for oestrogen metabolites), medical history, lifestyle and diet. In cases of a presence of a combination of high-risk genotypes, a hormone substitution therapy will not be prescribed: not conventional HRT, and not even bioidentical HRT (BHRT; which is considered safer and more efficacious than synthetic counterparts)28. In fact, dietary and lifestyle changes, and botanicals as supplementation, would be prescribed to decrease the risk of hormone-sensitive cancers. This is often given for minimally- to moderately-increased risks where the decision to prescribe BHRT is made on the basis of favourable oestrogen metabolism determined by genetic testing, a good 2-OH : 16α-OH ratio, absence of oestrogen dominance, and a balanced oestrogen : progesterone ratio.
Nutrigenetics to modulate oestrogen metabolism
Certain nutrients can selectively alter gene expression involved in oestrogen metabolism, modifying the type and amount of oestrogen metabolites. The aim is to enhance production of protective metabolites in order to safely prescribe BHRT. Once genetic testing is complete, there is a protocol for the application of nutrigenetics. Nutritional therapy can be used to balance oestrogen in the body and to deal with excess oestrogen from all resources as following:
- ■ Promote a healthy ratio of phase I oestrogen metabolites (2-OH : 16α–OH estrone)
- ■ Protect cells from Phase I oestrogen metabolites (carcinogenic 16α–OH and 4-OH estrone)
- ■ Promote healthy methylation (Phase II)
- ■ Encourage healthy intestinal excretion
- ■ Balanced (endogenous and exogenous)
oestrogen input.
Promote a healthy ratio of Phase I oestrogen metabolites
A number of lines of evidence support the conclusion that indole-3-carbinol (I3C) induces hepatic CYP4501A1 with a resultant increase in 2-hydroxyestrogens. Cruciferous (Brassica) vegetables and their glucosinolates — mainly I3C and DIM — showed extensive anti-oestrogenic effects, favourably affecting the balance of oestrogen metabolites towards protective 2-OH, and even inhibiting breast cancer cell growth in vitro.
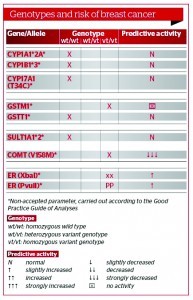
Figure 5 Combination of genotypes GSTM1 and COMT with an increased risk for sporadic breast cancer (predictive activity of enzymes, absent and decreased)
In this study, 18 volunteers received 500 g of fresh broccoli every day for 12 days, following a 6-day period of standard diet. The activity of CYP1A2, CYP2E1, E2 and 16α-hydroxylation were determined before and after the broccoli diet. The average activity of CYP1A2 and the average E2 : 16α-hydroxyestrone ratio increased by 19% (P < 0.0005) and 29.5% (P < 0.05) respectively29. This healthy ratio could also be promoted by adding flaxseeds on a daily basis. It has been found that as little as 25 g ground flaxseed leads to favourable shifts in oestrogen metabolites. Urinary concentrations of 2-hydroxyestrone, but not of 16a-hydroxyestrone, increased significantly in the flaxseed group. In the flaxseed group, the ratio of 2-hydroxyestrone to 16a-hydroxyestrone was positively correlated with urinary lignan excretion30.
There have been many controversies as to whether dietary soya should be used in breast cancer. As it increases the 2-0H : 16-0H ratio, which is a favourable oestrogen metabolism protective against breast cancer, this study is pro-dietary soya. The ratio of 2-OH estrone to 16a-OH estrone was higher during the isoflavone-rich soya diet compared with during the isoflavone-free diet, which saw a 27% increase31. One must be careful not to induce too much CYP1A1 owing to the risk of poor methylation as a result of a lack of supporting nutrients for healthy methylation and the presence of the COMT genotype with decreased predictive activity, as even ‘good’ metabolites such as 2-OH need to be methylated to prevent oxidation to more dangerous semiquinones. This is even more dangerous in the presence of exogenous toxins using the same CYP1A1 for detoxification, such as polycyclic aromatic hydrocarbons from fuel exhaust or chargrilled meat, and tobacco smoking for example. In practice, this means obtaining a fine balance between prescribed BHRT, supportive supplements and necessary lifestyle changes for safer hormone supplementation.