Antonino Araco and Francesco Araco discuss their prospective randomized study on the clinical efficacy and safety of highly purified polynucleotides and cross-linked hyaluronic acid for moderate to severe nasolabial folds

ANTONINO ARACO, MD, PhD,
Plastic Surgeon, Jenevì Medical Group, Milan, Italy; FRANCESCO ARACO, MD, phD, Aesthetic Doctor, San Giovanni Evangelista Hospital, Tivoli, Rome, Italy
email: [email protected]
Facial ageing is related to various tissue changes in different skin layers, such as dermal atrophy, loss of elastic tissue in the sub-epidermal elastin fibre network, and loss of deep fat1,2. There is a progressive loss of the mandibular profile and the nasolabial folds (NLFs) become deeper.
Hyaluronic acid (HA) plays a pivotal role in the control of tissue hydration and permeability to small or large molecules, and these properties contribute to its excellent biocompatibility and immune barrier function3. In the human dermis, the high percentage of HA allows for hydration, which at the same time maintains proper tissue volume — buffering skin cells from mechanical damage4–5. Alone or in combination with other molecules, such as polynucleotides, antioxidants, and glycosaminoglycans precursors, HA can also accelerate in vitro processes related to wound healing and in vivo tissue regeneration (e.g., burns, ulcers)6.
Polynucleotides Highly Purified Technology (PN-HPT™) is a compound containing a mixture of DNA polymers of different lengths. PN-HPT™ is obtained from male salmon trout gonads through purification and high temperature sterilization. PN-HPT™ has been used in aesthetic medicine as a biorevitalizer in monotherapy7 or associated with CO2 laser treatment for stretch marks8. It has also been used in dermatology for healing of venous ulcers of the lower limbs9, in postmenopausal women as biorevitalizer for the labia majora10, and in orthopedy for intraarticular osteoarthropathy knee treatment11. In this study, we evaluated the clinical association of PN-HPT™ with a subsequent HA dermal filler injection for the correction of moderate to severe NLFs.
Methods
This study was conducted in accordance with the ethical principles of the 1975 Declaration of Helsinki and is consistent with Good Clinical Practice principles.
Study population
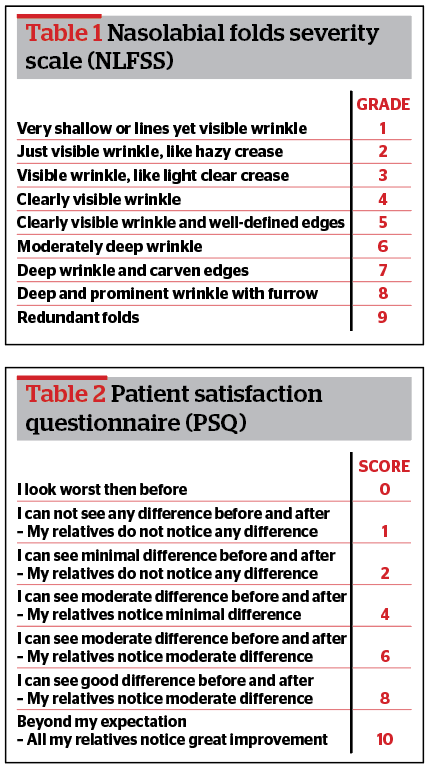
We included Caucasian women aged 40–65 years old with grade 7–9 (severe) NLFs on the validated 9-points Severity Scale (NLFSS)12 (Table 1). All were nonsmokers who had not undergone any dermal filler or dermal treatment for the last 12 months. We excluded men; Asian and African women; patients younger than 40 or older than 65; smokers; those with grade 1–6 NLFs according to the NFLFSS; subjects who had undergone any antiwrinkle/volumizing agent treatment in facial regions in the last 12 months; those with an uncontrolled disease, active inflammation, infection or lesions in the NFL area; subjects with a tendency for developing keloids or hypertrophic scars; those with active use of anticoagulant or antiplatelet medications; and patients with a history of autoimmune disease, chronic drug, or alcohol abuse.
Study design
Patients were divided and randomized into two groups: right nasolabial fold group (NLF Rx group) and left nasolabial fold group (NLF Lx group).
Stage one
All patients received 2 ml of intradermal PN-HPT™ (Plinest®, Mastelli, Sanremo, Italy) on the right NLF (NLF Rx group) and 2 ml of normal saline on the left NLF (NLF Lx group). A total of ten microdrops (0.2 ml at each injection point) were delivered by a 30 G, 8 mm needle. Patients did not receive any post-treatment medication and were asked not to massage the treated areas. The treatment was repeated after 3 weeks. In total, patients in the NLF Rx group received 4 ml of intradermal PN-HPT™ on the right NLF and patients in the NLF Lx group received 4 ml intradermal normal saline on the left NLF.
Stage two
After 3 further weeks, all patients received a total of 2 ml of subdermal cross-linked HA (Triplest n.3®, Mastelli, Sanremo, Italy). HA (1 ml) was delivered by a 27 G, 30 mm cannula in a retrograde manner on the right NSL and 1 ml on the left NLF. Patients did not receive any post-treatment medication and were asked not to massage the treated areas. The treatment was repeated after 3 weeks. In total, patients received 2 ml of subdermal cross-linked HA on the right NLF and 2 ml of subdermal cross-linked HA on the left NLF. The study was conducted from January to June 2020.
Assessment of efficacy and tolerability
Digital macro photographs were taken to ensure reproducibility in terms of positioning and lighting using a Nikon camera (Nikon Corporation, Tokyo, Japan; D7100, 12.0 megapixels, AF-5 micro Nikkor 60 mm, close-up 4D–62 mm + Nital Macro Lighting Spider). Furthermore, an assessment was made by Antera 3D® (Miravex Limited, Dublin, Ireland), which provides qualitative and quantitative analysis of wrinkles, texture, and haemoglobin. Antera 3D® has been proven to be effective and validated in treating wrinkles and analyzing texture13,14. Vectra H2® (Canfield Scientific, Wood Hollow Road, Parsippany, NJ 07054, United States) provided two unique sets of ranging lights to ensure optimal capture distance and resolution and to enable fine-scale 3D biomechanical analysis. This quantitative approach to assessing soft tissue changes assesses the degree of stretch, compression, and lift15.
Finally, patients were provided with a patient satisfaction questionnaire (PSQ) developed in-house by our working university group to measure patient satisfaction after comparing pre- and post-treatment photographs at 1- and 3-month follow-up (Table 2). The PSQ was anonymous. The questionnaire was administered by email, and all patients returned the questionnaire. Follow-up was performed after 6 weeks, 3 and 6 months. Results at 6 weeks, 3 and 6 months were compared to the baseline, and the results at 6 months were compared to the results at 3 months and 6 weeks.
Study endpoints
The first study endpoint was the improvement in dermal quality after PN-HPT™ versus placebo according to Antera 3D® at the 6-week follow-up.
The second endpoint was the reduction of NLFs by the injection of 4 ml of subdermal cross-linked HA assessed by one blinded independent doctor (F.A) from the Antera 3D® and Vectra H2® before and after treatment images (at 3 and 6 months).
The third endpoint was the interaction of the intradermal PN-HPT™ with the subdermal cross-linked HA on the duration of the dermal-filler gel as assessed by Vectra H2® at 3 and 6 months after treatment.
The fourth endpoint was the patient’s responses to the Patient Satisfaction Questionnaire (PSQ), a 10-point scale assessed before and after treatment (3 and 6 months) after a comparison of pre- and post-treatment photographs.
Assessment of safety
The patients’ faces were examined for any evidence of an acute response, such as erythema or oedema, and in the following days, any minor or major side-effects were recorded.
Statistical analysis
Data analysis was performed using the Statistical Package for the Social Sciences Windows version 13.0 (SPSS, Chicago, Illinois, USA). Descriptive statistics for quantitative continuous variables were the mean and standard deviation after confirmation of a normal distribution. Normality assumptions were demonstrated with histograms, Q-Q plots, skewness, and kurtosis, as well as the Kolmogorov/Smirnov and Shapiro-Wilk tests. Descriptive statistics for qualitative categorical variables were performed using frequencies. Student’s t-test was used to compare continuous variables among groups. The χ2 test and Fisher’s exact test were used to compare nominal variables. All p values were considered significant if below 0.05.
Results
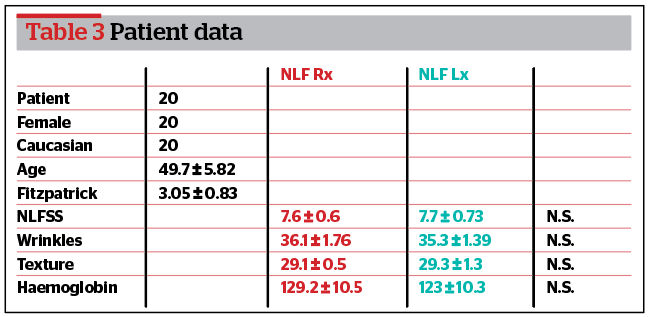
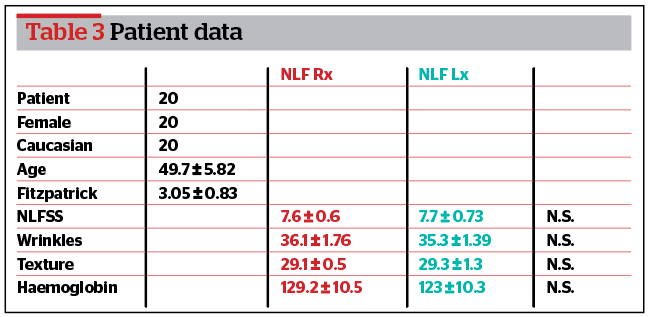
Twenty women (average age 49.7 ± 5.82 years, range 45–60 years) who attended the author’s aesthetic medicine practice in Milan and who fit the inclusion criteria were enrolled in this study (Table 3). Before treatment, they were asked to stop using any cosmetic creams, make-up, or sunscreen creams and to avoid exposure to the sun for 4 weeks before and during the study period. Patients were informed about the study treatment protocol and signed a consent form. They received the full treatment according to the study design. No minor or major side‑effects were reported during the study, and all patients completed the follow-up after 6 months.
Results in the NLF Rx group
According to Antera 3D®, wrinkles (p<0.05) and skin texture (p<0.05) significantly improved at 6 weeks, 3 and 6 months after treatment compared to the baseline. Haemoglobin significantly improved at 3 and 6 months after treatment compared to baseline but did not improve at 6 weeks (p>0.05). Based on the PSQ data, patients were satisfied at 3 months and moderately satisfied 6 months post-treatment.
Results in the NLF Lx group
According to Antera 3D®, wrinkles (p>0.05), skin texture (p>0.05), and haemoglobin (p>0.05) failed to improve at 6 weeks and 6 months after treatment. Wrinkles (p<0.05) and skin texture (p<0.05) significantly improved at 3 months of treatment compared to baseline. Based on the PSQ data, patients were satisfied at 3 months post-treatment.
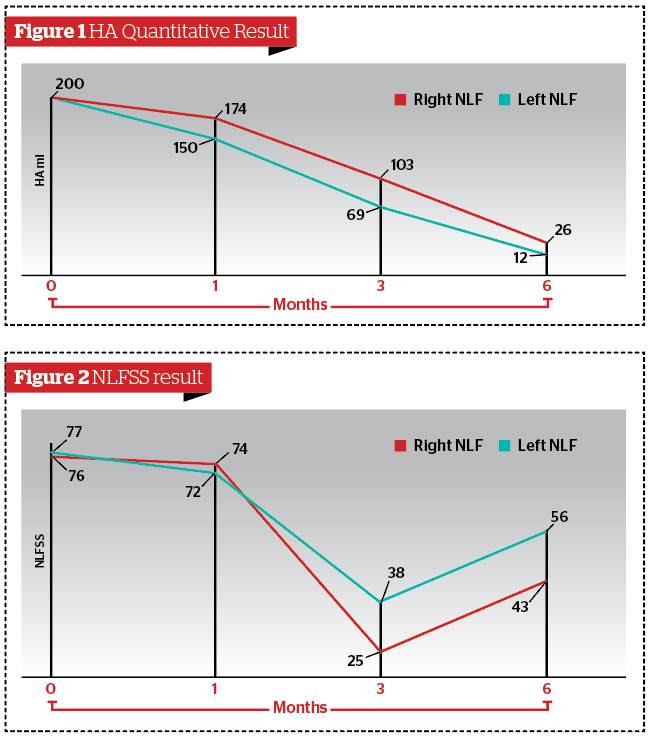
HA quantitative result and NLFSS
According to Vectra H2®, the amount of HA on the right NLF was significantly higher than the left NLF either at 3 (p<0.05) and 6 months (p<0.05) (Figure 1). According to the NLFSS, the reduction of the right NLF was significantly higher than the left NLF either at 3 (p<0.05) and 6 months (p<0.05) (Figure 2).
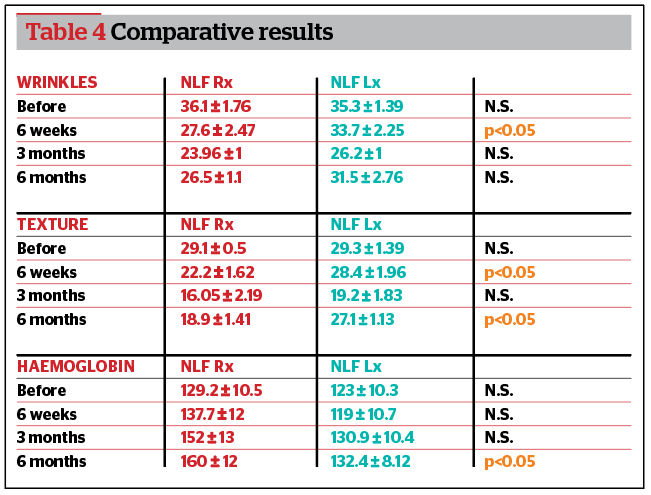
Comparative results among groups
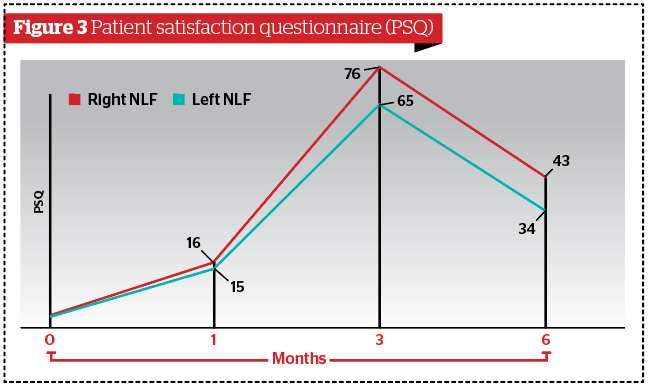
According to Antera 3D®, wrinkles were significantly more improved in the right NLF group (p<0.05) compared to the left NLF 6 weeks after treatment. Skin texture was significantly more improved in the right NLF group (p<0.05) compared to the left NLF at 6 weeks and at 6 months after treatment. Haemoglobin improved significantly more in the right NLF group (p<0.05) compared to the left NLF 6 months after treatment. HA duration and NLFSS were significantly higher in the right NLF group (p<0.05) compared to the left NLF at 3 and 6 months after treatment (Table 4). No difference was recorded for PSQ (p>0.05) (Figure 3).
Discussion
Nasolabial folds (NLFs) are the first and most evident sign of facial ageing16,17. HA replacements have been used in aesthetic medicine practice since the early 1980s for the correction of wrinkles and NLFs18,19. Currently, many dermal HA-based medical devices are used in aesthetic medicine, most of which are based on chemical cross-linking. This chemical modification improves stability, rigidity, and elasticity (elastic modulus G’ and viscous G”), but substantially modifies the natural molecular structure20,21. Polynucleotides Highly Purified Technology (PN-HPT™) (Mastelli Srl, Sanremo, Italy) is a compound containing a mixture of DNA polymers of different lengths. PN-HPT™ is obtained from the sperm of trout or salmon using an extraction process in which purification and high-temperature sterilization procedures are performed to obtain a pure active product. Numerous studies have shown that PN-HPT™ injections can be used in clinical settings across a wide range of medical specialties7–11. Recently, specific guidelines have been implemented regarding the use of PN-HPT™ in aesthetic medicine and dermatology22. In a recent preliminary prospective and randomized study, we showed that PN-HPT™ as monotherapy is safe and effective for the treatment of atrophic acne scars compared with placebo23.
The goal of this current study was to clinically investigate the interaction of PN-HPT™ and HA. Our hypothesis was that PN-HPT™ could be a substrate for HA and could enhance its clinical efficacy and duration. For that reason, we tested a combined treatment of PN-HPT™ and HA on NLFs in a split face pattern and compared the PN-HPT™ group with a homogeneous control group at 6 weeks, 3 and 6 months. In this study, we only included Caucasian women and excluded other groups in order to avoid ethnicity bias.
By using Antera 3D®, we demonstrated changes in the quality of the dermis and, in particular, qualitative and quantitative analyses of wrinkles, texture, and haemoglobin.
This study showed that PN-HPT™ significantly improved wrinkles and skin texture at 6 weeks and 6 months compared with the control placebo group. In fact, at 6 weeks only the NLF Rx group received PN-HPT™. Those results confirmed clinical data we had already reported23. At 6 months, the HA reabsorbed in the right NLF still had an enhanced effect on dermal quality due to PN-HPT™.
By using Vectra H2® we showed that the amount of HA on the NLF Rx group was significantly higher than the left NLF either at 3 and 6 months. This suggests that on the right NLF, the HA was reabsorbed less because of the substrate effect of the PN-HPT™. Furthermore, the reduction of the clinical appearance of the right NLF was significantly higher than the left NLF either at 3 and 6 months, according to the NLFSS.
Additionally, we found that haemoglobin was improved significantly at 6 months after treatment only in the NLF Rx group suggesting that PN-HPT™ improved the vascularity of the targeted tissue. Finally, the PSQ reflected the clinical results. In fact, at 3 months, all patients were satisfied because of the HA effect, but at 6 months only those patients in the NLF Rx group were still moderately satisfied. No complications were recorded after the treatments, which suggests that the PN-HPT™ and HA treatments are safe.

We believe that the results of this study are promising. In fact, PN-HPT™ alone has been proven to be effective as a derma bio-stimulant and is proven to be a substrate for HA duration and efficacy.
The present study has some limitations. First, the number of patients enrolled in this study was small; however, we found it difficult to enrol more patients due to the strict inclusion criteria.
Second, we used a split face randomization study design, but a double-blind randomization scheme with a much higher statistical power would be necessary to confirm our results. Finally, in order to confirm our hypothesis that PN-HPT™ could work as a dermal substrate, further clinical studies with a larger cohort and with a wider range of materials (HA, calcium hydroxylapatite) would be necessary.
Conclusions
Our prospective and randomized clinical study showed that PN-HPT™ monotherapy improves dermal quality and enhances the clinical efficacy and duration of cross-linked HA for the treatment of moderate to severe nasolabial folds.
Declaration of interest None
Figures 1-3 © Antonino Araco
Tables 1-4 © Antonino Araco
References
- Gierloff M, Stohring C, Buder T, Gassling V, et al. Aging changes of the midfacial fat compartments: a computed tomographic study. Plast Reconstr Surg 2012;129:263–73.
- Le Louarn C, Buthiau D, Buis J. Structural aging: the facial recurve concept. Aesthet Plast Surg 2007;31:213–8.
- Beasley KL, Weiss MA, Weiss RA. Hyaluronic acid fillers: a comprehensive review. Facial Plast Surg 2009;25:86–94.
- Dicker KT, Gurski LA, Pradhan-Bhatt S, Witt RL, Farach-Carson MC, Jia X. Hyaluronan: a simple polysaccharide with diverse biological functions. Acta Biomater 2014;10(4):1558–70.
- Vigetti D, Karousou E, Viola M, Deleonibus S, De Luca G, Passi A. Hyaluronan: biosynthesis and signaling. Biochim Biophys Acta 2014;1840(8):2452–9.
- Hiramoto K, Kobayashi H, Yamate Y, Ishii M, Sato EF. Intercellular pathway through hyaluronic acid in UVB-induced inflammation. Exp Dermatol 2012;21(12):911–4.
- Cavallini M, Papagni M. Long Chain Polynucleotides Gel and Skin Biorevitalization. International Journal of Plastic Dermatology-ISPLAD. 2007; 3:27-32.
- Gianfranco Matera, Nicholas Dodici, Mauro Raichi Improving on laser: biorevitalization of stretch marks, the polynucleotides infiltrations combined with CO2 laser option. Aesthetic Medicine 2020; Volume 6 / Nº2 / April .
- G. De Caridi, M. Massara, I. Acri, S. Zavettieri, R. Grande, L Butrico, S. de Franciscis, R. Serra. Trophic effects of polynucleotides and hyaluronic acid in the healing of venous ulcers of the lower limbs: a clinical study. Int Wound J 2016; 13(5):754-8.
- I. P. Palmieri, M. Raichi. Biorevitalization of postmenopausal labia majora, the polynucleotide/hyaluronic acid option. Obstet Gynecol Rep 2019; 3: 2-5.
- Giarratana L.S., Marelli B.M., A randomized double-blind clinical trial on the treatment of knee osteoarthritis: The efficacy of polynucleotides compared to standard hyaluronian viscosupplementation. The Knee 2014; 21:661-668.
- Jiechen Z., Wei Hou,. Classification of facial wrinkles among Chinese women. J Biomed Res 2017;31 (2):108-115.
- Dissanayake B, Miyamoto K, Purwar A, Chye R, Matsubara A. New image analysis tool for facial pore characterization and assessment. Skin Res Technol 2019; 25:631-638.
- Jang SI, Kim EJ, Park H, Kim HJ, Suk JM, Kim BJ, Lee JH, Lee HK. A quantitative evaluation method using processed optical images and analysis of age-dependent changes on nasolabial lines. Skin Res Technol 2015; 21:201-6.
- Tanizaki. H et al., Quantitative evaluation of atrophic acne scars using 3D image analysis with reflected LED light Skin. Res Technol 2019; Sep 2.
- Baumann L. Skin ageing and its treatment. J Pathol 2007;211(2):241–51.
- Ezure T, Amano S. Involvement of upper cheek sagging in nasolabial fold formation. Skin Res Technol 2012;18:259–64.
- Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid: A key molecule in skin aging. Dermato-endocrinol 2012. 1;4(3):253–8.
- Ascher B, Bayerl C, Brun P, Kestemont P, et al. Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of severe nasolabial lines – 6-month interim results of a randomized, evaluator-blinded, intra-individual comparison study. J Cosmet Dermatol 2011;10:94–8.
- Croce MA, Dyne K, Boraldi F, Quaglino D Jr, Cetta G, Tiozzo R et al. Hyaluronan affects protein and collagen synthesis by in vitro human skin fibroblasts. Tissue Cell 2001;33(4):326–31.
- La Gatta A, Schiraldi C, Papa A, De Rosa M. Comparative analysis of commercial dermal fillers based on crosslinked hyaluronan: Physical characterization and in vitro enzymatic degradation. Polym Degrad Stab 2011;96(4):630–6.
- M. Cavallini, E. Bartoletti, L. Maioli, A. Massirone, I. P. Palmieri, M. Papagni, M. Priori, G. Trocchi, members of The Polynucleotides HPT™ Priming Board, Italian College of the Aesthetic Medicine Scientific Societies)— SIME, AGORÀ, SIES Consensus Report on the Use of PN-HPT™ (Polynucleotide Highly Purified Technology) in Aesthetic Medicine Journal of Cosmetic Dermatology 2020; August 16.
- Araco A, Araco F. Preliminary prospective and randomized study of highly purified polynucleotide versus placebo in treatment of moderate to severe acne scars. Aesthetic Surgery Journal 2021;