Dexlevo unveil INIBLANC, their patented solubilized, biocompatible and biodegradable PCL

There are many hyaluronic acid-based dermal fillers and polymer products available on the market. However, many are mainly focused on localized areas for a volumizing effect. Furthermore, we cannot overlook the possibility of side-effects by blocking blood vessel or pressing them.
INIBLANC is a new type of injectable based on fully solubilized biocompatible and biodegradable PCL, which spreads naturally into the surrounding area of skin. Thanks to DEXLEVO’s unique CESABP Technology, you can apply INIBLANC safely and easily for the entire FACE LIFT.
CESABP(Collagenesis-Enabled Solubilized Active and Biodegradable Polymer) is DEXLEVO’s patented technology for manufacturing fully solubilized biocompatible and biodegradable PCL.
Benefit 1: Spreadability
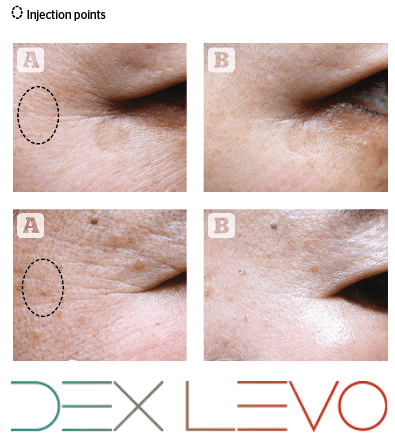
(B) around the eyes
12 weeks after injection.
We applied 0.7ml of INIBLANC injecting just one point around eyes to see how it spreads. Each of the around eye was photographed at 12 weeks after application. Around the eye such as eye crow’s feet and elasticity of each patients was improved by only one point application. Since the fully solubilized liquid PCL of INIBLANC spreads smoothly and widely into the skin.
Benefit 2: Collagenesis
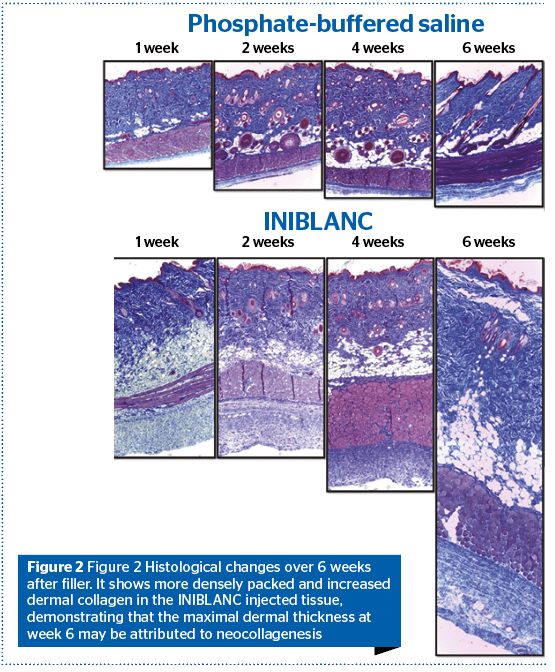
To evaluate neo-collagenesis, biopsy specimens of 6 week-old female Sprague Dawley rat were obtained at 1,2,4, and 6 weeks after the INIBLANC injection. While the expression level of collagen upon MT staining in the PBS-injected group increased only slightly over time, the INIBLANC injected group showed a marked increase during the first 6 weeks. The degree of dermal thickening of PBS injected group did not induce clinical distinction, compared to INIBLANC.
Biopsy specimens from the INIBLANC, however, showed increased thickness of the dermis since the first week, sparing that of the subcutaneous fat. In addition, the volume expansion and stretching are achieved by the activation of fibroblasts.
Benefit 3: Wrinkle improvement
Injection of up to 1ml was injected into the intramuscular cavity of 29 subjects to evaluate wrinkle improvement. Independent evaluators and testers assessed the CFGS(Crow’s Feet Grading Scale) for each of the application areas of INIBLANC and competitor at 2 weeks, 4 weeks, and 12 weeks after the final application.
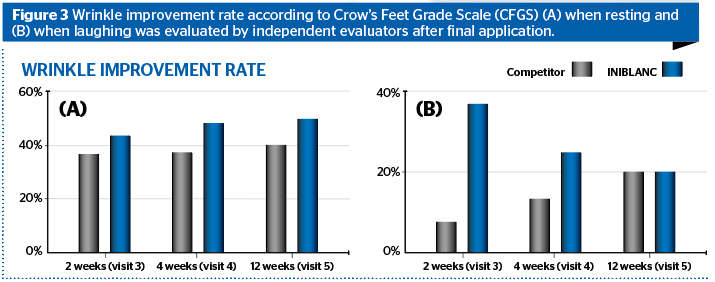
The CFGS (Crow’s Feet Grading Scale) when Resting and when Laughing was evaluated by the independent evaluator at 2, 4, 12 weeks after the final application of INIBLANC and competitor (other medical device).
Four weeks after the final application of the INIBLANC, the improvement rate of the Resting-CFGS was 48.28% comparing to 41.38% of control group. Four weeks after the final application of the INIBLANC, the improvement rate of the Laughing-CFGS, was 20.69% comparing to 13.79% of control group.
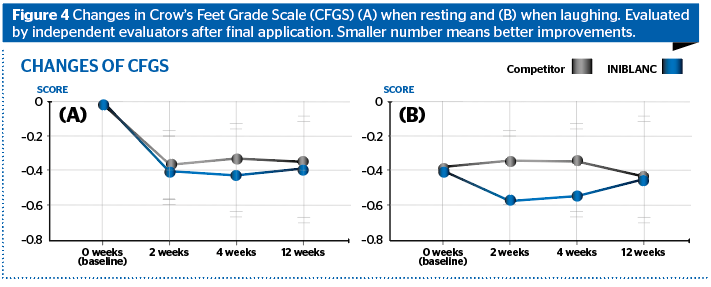
Changes in CFGS (Crow’s Feet Grade Scale) when resting (A) and when laughing (B) was evaluated by independent evaluators after final application. The average of both CFGS scores of INIBLANC for the Resting and Laughing group have shown more efficient results compared to competitors.
Conclusion
INIBLANC, a new type of injectable based on the world’s first Fully Solubilized PCL without micro particle, encourages face lifting and elasticity for entire face by neo-collagenesis. Unlike other existing filler products, it improves entire face safely and effectively without any side effects.
Find out more at: dexlevo.com




