Jan Balczun offers an insightful overview of the fillers available on the market, guidance on the things that can go wrong and, more importantly, what you should do next
public awareness and acceptance of minimally invasive aesthetic procedures is continuously on the rise; particularly with the new generation of dermal fillers, there are results achievable that were previously only possible through costly surgery with unsuitable social downtime and intensive postoperative care.
Facial rejuvenation procedures have experienced enormous growth, the number of procedures for soft tissue augmentation with dermal fillers was 2.6 million in 2016 in the US, which is an increase of 2% in comparison to 2015 and an increase of 298% from 2000. Two million of these procedures were performed using hyaluronic acid, which is another increase of 3% compared to 20151.

With the growth of the market and the increasing demand from patients for new indications and possibilities, it is hard to keep track with the types of filler available on the market and the potential risks.
For this reason, after touching on the available products, this article will give an overview and guidance on all the things that can go wrong with dermal fillers.
Classification of dermal fillers
Non-biodegradable products
Silicone
Silicone was injected for more than 40 years as an oil containing polydimethylsiloxane polymers, which were made from silicon, oxygen and methane molecules. This fluid had a viscosity of 350 centistokes.
There is no report of allergy to silicone in the medical literature, and it has the least reactivity of foreign body materials10. The injection technique for silicone was the micro-droplet technique at monthly intervals11.
The augmentation by silicone was due to a combination of dermal connective tissue displacement in combination with collagen capsules around the silicone droplets.
There are reports in the literature of delayed inflammatory nodules even years after injection12, collagen fibrosis and, due to impurities of the silicone, associations with foreign bodies in the cytoplasm of multinucleated cells13. Other reports describe lymph vessel blockage, rosacea-like reactions, migration, delayed hypersensitivity, surface deformities, embolism, and even blindness14–18.
These and the uncertain long-term risks led to the ban of silicone (350 cs) as a dermal filler in most countries worldwide.
The only available injectable silicone is not FDA approved for tissue augmentation but rather for ophthalmic use. (Silikon 1000 [Alcon, Fort Worth, TX] and Adaptosil 5000 [Bausch Lomb, Rochester, NY]). The higher centistokes indicate a higher viscosity and make it harder to depress through a small needle.
Polymethylmethacrylate
Originally marketed as Artecoll (Rofil Medical International B.V., Breda, the Netherlands) in Europe and Canada in 1993, it is known as Artefill in the US. It was FDA approved in 2007 and consists of round and smooth polymethylmethacrylate microspheres of 30 to 42 μm diameter19, which are surrounded by bovine collagen. Due to the bovine origins, it is necessary to perform an allergy skin test prior to injection20.
Numerous reports show the occurrence of severe adverse reactions21, with most of these reactions taking place months to years after the injection and presenting as delayed persistent granulomas. Most commonly, these granulomas appear around the lip after lip augmentation22. These late onset granulomas show typical histological aspect23,24 corresponding to PMMA.
The effect is permanent due to the replacement of the bovine collagen with host connective tissue within three months. This permanence again requires a well trained and experienced injector.
Polyacrylamide
Products containing polyacrylamide must not be degraded, as these monomers of acrylamide are toxic. The toxicity of acrylamide is due to its capability of transecting neurons and causing an axonopathy25. Examples are Evolution, Outline (both Procytec, France), and Aquamid (Contura SA, Switzerland).
Biodegradable products
Calcium hydroxylapatite
Compared to HA the amount needed to smooth the wrinkles is lower with CaHA and the duration is longer, up to 15 months30.
Due to the report of unacceptable high numbers of nodules, CaHA is not recommended for lip augmentation31.
Poly-l-lactic acid
Poly-l-lactic acid is a major component of Vicryl sutures (Ethicon Inc, Sommerville, NJ) and was formulated and marketed as a dermal filler in 1999 in Europe under the name ‘New Fill’. Its particles are about 40–63μm in size and suspended in a sodium oxymethycellulose carrier. It was approved by the FDA in 2004 and named Sculptra (Valeant, West Laval, QC, Canada) in 2004. The first indication for PLLA was HIV-associated lipodystrophy.
Its method of action is to cause a formation of multinodular gant cells in the subcutaneous soft tissue32. Due to this effect, the final result is only gradually achieved, because PLLA fillers induce an increase in (sub)dermal thickness. The PLLA is degraded through a metabolization process that first converts the PLLA into lactic acid monomers and finally into glucose and CO233,34.
A common side-effect of PLLA is product related nodules at the injection site, especially in the population with HIV-associated lipodystrophy. Clinical studies defined these lesions as typically palpable, asymptomatic, non-visible and 5 mm or less in size35,36. In two different studies on HIV-related lipoatrophy with separate patients populations in Europe, these product related nodules appeared in 52% (26/50) and 31% (9/29) of cases37.
The pain while injecting is relatively high, the costs are higher than comparable products, and the results are only shown in a delayed onset, but due to its non-immunogenic character, no skin testing is necessary.
Collagen
Due to its animal origin, bovine collagen can be immunogenic (Zyderm1, Zyderm 2, Zyplast [Allergan, Irvine, CA]). This explains the necessity for two skin tests within an interval of two weeks prior to injection. The first skin test should be placed in the antecubital area, while the second test is usually performed on the facial skin, preferably in a non-visible area such as the scalp line. The positive first reaction rate among patients is usually around 3.5%, with a manifestation rate of 70% within 48–72 hours38. This risk can be lowered to 0.5% by performing a second test. Some series show an incidence of foreign-body-granulomas in no less than 1.3% of patients39.
CosmoDerm 1, Cosmoderm 2, and Cosmoplast (Allergan, Irvine, Ca) are from a human collagen source, therefore non-immunogenic.
Evolence (ColBar LifeScience Ltd. Rehovot, Israel) is made from purified porcine collagen and was approved by the FDA in 2008. Even though this filler is derived from an animal source, there is no need for skin testing prior to injection due to its very low potential for hypersensitivity40,41. But it should not be injected in patients with a positive history of porcine allergy. The effect of collagen derivated fillers such as Evolence last for at least 6 months42 and histopathological analysis of Evolence in vivo showed a growth and migration of fibroblasts one month after injection43.
Hyaluronic acid
Hyaluronic acid is a glycosaminoglycan polysaccharide composed of alternating d-glucuronic acid and N-acetyl-d-glucosamine monosaccharides44. These sugars are present in the human body with no species specificity and its functions in the human body include space filling, shock absorption, and protein exclusion among other tasks.
It is degraded in the human body after injection in 24-72 hours. To make it longer lasting, it is cross linked by binding proteins. The more cross-linked a hyaluronic acid is, the longer its duration45. The remaining proteins must be washed out after the binding process. Remaining proteins may be a risk to hypersensitivity reactions46–49. Studies also showed anti-HA antibodies50, but the significance needs to be clarified. The injected and cross-linked product is absorbed by the surrounding tissue through a process called isovolumetric degradation51.
Due to its hydrophilic nature, HA is able to maintain its shape using the surrounding water in the soft tissue. One gram of hyaluronic acid can bind with up to 6l grams of water52.
Nearly all modern HA fillers are derived from bacterial fermentation from a Streptococcus species. Therefore, there is little risk of contamination with xenogeneic diseases, such as BSE or animal pathogens or allergens53.
Due to their water binding capacity, HA fillers should never be used with the technique of overcorrection. The results of HA fillers are almost immediate with very good predictability, the risk is only minimal and there is no downtime after injection.
These reasons make the injection with HA fillers one of the most sought after procedures in the aesthetic field. Meanwhile, there are more than 160 different HA fillers from more than 50 different companies on the market in Europe.
Complications of dermal fillers
There are three main stages in which the adverse reactions to dermal filler can be related to: either the procedure itself, the procedural technique or the agent injected54. Of all these, the group with the most adverse reactions is the misplacement of dermal fillers in biodegradable products55, whereas material related complications are far more associated with permanent fillers.
To categorize the undesired side-effects of dermal fillers, they can be divided into mild, moderate, and severe complications.
Mild complications
Bruising
As one of the general risks of injections, the injection of any dermal filler has the potential of bruising. It is more frequently observed after injection with a fanning or threading technique in the dermal or immediate subdermal planes56, as many small cutaneous blood vessels can be found there. By using the depot technique or injecting into deeper planes of the skin, the risk of bruising is reduced. The number of injections should be limited and the smallest gauge needle possible used for injection. Precautions can be taken by the patient by avoiding all blood thinning medication57 (if possible) at least one week prior to the procedure, the patient should stay out of the sun as long as the bruising persists and vigorous exercise should be avoided for the first 24 hours.
To treat any bruising right after injection, the application of cold compresses or a vitamin K cream has proven to show good results58. In minor cases, the application of camouflage make-up is sufficient59, whereas longer lasting bruises might be treated with pulsed dye light or KTP lasers.
Edema
Post-traumatic edema, which occurs almost immediately after injection, is related to the volume injected and the technique used. This is a normal finding and may be treated like bruising and is in almost every case gone within a few hours or days.
Moderate complications
Immediate hypersensitivity reaction
As any dermal filler is a potential foreign body, the filler might be the target for a type I hypersensitivity reaction (immunoglobulin-E mediated immune response). These reactions appear right after injection with a delay of only minutes after exposure and this may occur during any injection, and a history of previous injection may not give false security. The method of action in a type one reaction is a degranulation of mast cells leading to a release of proteases, heparin, leukotrienes, and platelet-activating factor among other transmitters that result in the typical allergic indicators, such as itching, pain, erythema and edema. These signs can be mild to severe and last up to several weeks60.
As in any case of allergic reactions, the treatment depends on the severity of the reactions presented. The majority of swellings resolve spontaneously and are no longer visible after a short period of time. If the edema is mediated through mast-cells, it may respond well to antihistamines. With persistent edemas, the treatment should include oral steroids and the patient should be monitored very closely. If the edema is progressing, the patient has to be treated as a medical emergency to avoid airway obstruction. Some cases of anaphylactic shock have been reported61, but these cases are very rare62 and occur mostly in relation to bovine collagen. Care should also be given to a possible reaction to the anaesthetic within the filling substance.
Inappropriate placement
Fillers should always be placed in the suggested layer. If the placement is not in the correct layer, especially if it is placed more superficial than supposed, the result may be a blue hue in hyaluronic acid fillers (often referred to as the Tyndall effect) or — even worse — to visible lumps of the injected material63. The more superficial from the suggested layer the material is placed, the longer the duration of the discoloration64. As most fillers are biodegradable, especially the commonly used HA fillers, the blue hue will disappear with time and, therefore, treatment may not be necessary if it is only small and imperceptible.
Under aesthetic considerations and from the patient’s expectations, the ‘sit and wait’ approach is not an acceptable option65,66. In cases of inappropriate placement of filler material, the first step should be a vigorous local massage and, if needed, followed by incision and drainage. If the filler material is hyaluronic acid, a good first option is the use of hyaluronidase67. PMMA (e.g. Artecoll) is less forgiving if placed too superficial, leading to long-lasting itching and redness and, in rare cases, to hypertrophic scarring. These symptoms can be significantly reduced with intradermal steroids68 or pulse dyed laser application. For nodules resulting from superficial placement of CaHA (Radiesse) the treatment should always be incision and drainage69.
Skin discoloration
No matter which dermal filler is inserted into the skin, there is always the risk of skin discoloration. Some redness occurring almost immediately after injection is a typical side-effect and does typically resolve within a few days70. This is usually a result of the inflammatory response of the injection site. If the redness does not dissolve within a few days, this finding may conclude a hypersensitivity reaction. The treatment should follow the path of a rosacea- treatment with topical steroids and oral tetracycline or isotretinoin. The topical use of Vitamin K has also found to accelerate the resolution of erythema71.
Another risk at the injection site is excessive tissue expansion either by the product or excessive massaging by the injector. This may lead to a neovascularization, which usually fades within 3–12 months without treatment. If the neovascularization is bothersome for the patient, treatment with lasers has good results. The choice of laser depends on the size of vascularization and does include KTP-Laser at 532 nm, diode copper vapor laser at 585 nm, pulsed dye lasers at 585 nm and IPL (intense pulsed light).
In relation to HA fillers and the Tyndal effect, recent studies show that the blue hue is in fact not related to the Tyndall effect but to the reflective properties of the skin72. Because waiting for the product to dissolve is not an option, the initial approach should be to use hyaluronidase. If that proves not to be sufficient due to the high cross-linking of the HA, incision and expressing the filler can be performed73 for up to 12 months74.
The Cosmetic Surgery National Data Bank statistics stated that in 2013, 22% of all cosmetic procedures were performed on racial and ethnic minorities75. The coloured skin tends to hyperpigment after trauma, and as the injection of dermal fillers is a small trauma to the skin, post-inflammatory hyperpigmentation is a common problem after dermal filler procedures, especially in skin types IV-VI on the Fitzpatrick scale76,77. If hyperpigmentation occurs after dermal filling, the first step as a treatment should be bleaching agents (e.g. topical hydroquinone) together with Retin-A and strong daily sunscreen. If this is not helpful, the next treatment should include IPL and laser-techniques. To minimize the risk of post-inflammatory hyperpigmentation, the number of injection sites should be limited.
Malar edema
This complication may occur with any filling substance when used to fill the infraorbital hollow as well as the tear trough78.
Twelve out of 51 patients (23%) who received intraorbital hollow augmentation with HA had prolonged edema for an average of 5.4 months in a retrospective study79. In a study on fresh cadaver dissections, Pessa and Garza80,81 were able to identify the malar septum, a fascial structure leading from the orbital rim at the arcus marginalis superiorly towards the cheek skin roughly 3 cm inferiorly the to the lateral canthus. The malar septum divides the SOOF (suborbicularis oculi fat) in a superficial and deep compartment. While the lymphatic drainage of the deep compartment flows into the cheek fat82, the superficial compartment only has compromised lymphatic drainage.
This lymphatic barrier is important to keep in mind; by injecting superficially to the malar septum, the barrier is augmented, making it impermeable to lymphatic fluid, resulting in fluid accumulation and malar edema.
As it responds only poorly to any conservative therapy, hyaluronidase should be injected to dissolve the compromising HA.
To avoid malar edema, the injection has to be placed in the deep compartment and the substance used should be hyaluronic acid.
Nodular masses
If a nodular mass develops after injection, the initial assessment needs to include the impact the treatment would have on the patient. There are, for example, some nodules palpable but not visible and, therefore, best left with a ‘watch and wait’ line of therapy, while others require immediate action. Usually, these delayed nodular masses present after weeks or months and can be distinguished as either non-inflammatory or inflammatory nodules.
Delayed non-inflammatory nodules
Non-inflammatory nodules are considered to be one of the most common adverse events after dermal filling procedures83. They have a firm, discrete and regular surface with no signs of erysipelas or fever. The most common cause of non-inflammatory nodules is product misplacement or migration.
The initial management depends on the material injected. With HA products, hyaluronidase is the treatment of choice. With more lasting substances like CaHA another valuable option is to disrupt the product with either saline or lidocaine injection followed by vigorous massaging84. Non-resolving nodules can be treated with small amounts of intralesional steroids to prevent atrophy of the skin.
Between the close follow-up appointments the patient should be told to massage at home to improve the dissolving process.
If the nodules persist for several months and do not respond to any treatment, they will become increasingly fibrotic and, in this case, will require excision.
Delayed inflammatory nodules
Usually, inflammatory delayed nodules present as painful, tender and have a reddish colour85.
In the literature, there is a considerable debate whether these delayed inflammatory nodules are the result of biofilms86. Biofilms occur when a material is injected into the subdermal skin and become coated with bacteria. The bacteria form a complex and secrete a protective (and also adhesive) matrix so they can irreversibly adhere to either a living structure or an inter‑surface. The low-grade but chronic infection is almost resistant to antibiotics87, 88.
As it is hard to distinguish a delayed inflammatory nodule from a low-grade hypersensitivity reaction, any red indurated area regardless of duration should be considered as a biofilm89,90.
Treatment should start with an antibiotic, either macrolide or tetracycline91 for at least 14 days and the affect then needs to be re-evaluated. If there is significant improvement at the follow-up appointment, the antibiotics should be continued for 4 more weeks. If no improvement is noticed, the therapy should be exceeded to a dual antibiotic therapy92.
If after 6 weeks there is still no improvement, the path of treatment divides between HA fillers and other substances injected. In HA fillers, treatments with hyaluronidase should begin93,94 and continue every 4 weeks until complete resolution of the nodules, while the antibiotic therapy continues.
Intralesional steroids are the treatment of choice if the injected substance was not an HA or the HA does not respond to hyaluronidase95, 96. The preferable steroid is triamcinolone acetonide 40mg/ml. As post-treatment soft tissue atrophy (20–30%) and telangiectasia97 is described, the patient needs to be aware of these side‑effects.
The last resort for any delayed nodule is surgical excision.
Foreign body granulomas
This is a very rare late complication and only appears in 0.1% of the patient population98. Most of the foreign body granulomas occur after the injection of permanent or semi-permanent fillers. This reaction of the body to foreign material presents singularly or as a small cluster in most of the cases as dermal nodules. Usually they appear within 6 months after injection but there are also reports in the literature of delayed granulomatosis after more than 14 months after injection of PMMA99.
Infection
As a procedure that breaks the natural barrier of the skin, a risk to dermal filling procedures is an infection of the treated area. To minimize this risk, general precautions, like in every procedure, should take place, such as proper disinfection and the usage of sterile products as well as gloves during the injection100.
If a diffuse inflammation of the injection site occurs, the common causative agent is Staph. aureus or Strep. pyogenes as a natural ingredient of the persistent skin colonialization. This infection might be misinterpreted as hypersensitivity reaction, but erysipelas is associated with fever and itchiness. Depending on the severity of the infection, antibiotics should be given either orally or through IV. It is not recommended to massage that area as this might lead to the infection spreading.
If the infection continues, it might lead to an abscess, which is a very rare complication. As with every abscess, it should be treated with incision and drainage in addition to antibiotics.
Herpes outbreak
If a patient reports a history of herpes infection, a virus reactivation may be provoked by injection due to direct damage to the axon by the needle101. Tissue manipulation or an inflammatory reaction after injection of dermal filler might be alternative causes of reactivation, but this needs to be investigated further. It has been demonstrated that the hyaluronic acid itself acts as a protective agent, preventing viral replication102.
If a reactivation of herpes infection occurs, it usually presents 24–48h after injection. The localization is usually the site of the dermal filler injection, most commonly in the nasolabial folds as well as the perioral area. An extension toward neighbouring areas is rare but might take place103.
The treatment is dependent on the severity of the outbreak. Antiviral therapy can be used, from ointment to IV administration.
Severe complications
Vascular compromise
As one of the worst side-effects of dermal fillers, this indicates a major and immediately occurring complication. Therefore, the recognition of a vascular event and swift and aggressive treatment is necessary to avoid serious, potentially irreversible complications104–109.
Complications reported in the literature, besides tissue necrosis and retinal artery occlusion (see below), are acute vision loss and hemiplegia after autologous facial fat injection as a result of ocular and cerebral embolism, respectively110–112, as well as acute fatal stroke that has also been reported after autologous fat injection into the glabella113.
Tissue necrosis
The mechanism of tissue necrosis after dermal filler injection can be divided into two distinct paths: the first relates to vascular compromise by injecting too much material too close to a vessel, leading to an interruption of vascular supply due to compression114. The other mechanism is the insertion of material directly into the vessel leading to a direct obstruction.
The first noticeable signs of an event of vascular compromise is a prolonged blanching, often including some pain, followed by a dusky discoloration after a certain period. Always be alert if the blanching persists for more than 30 minutes.
The regions most prone to vascular compromise are the regions with only minimal collateral circulation, e.g. the nose; areas with terminal blood supply, such as the glabella; or large vessels, such as the nasal artery. The terminal supratrochlear artery only has small branches and is responsible for a considerable skin area of the forehead with only minimal collateral circulation. Furthermore, the vascular anatomy in this region is unpredictable115, poor and predominantly terminal116. The majority (more than 50%) of the reported cases in the literature occurred in the glabella region117–122.
Retinal artery occlusion
When the force of injection exceeds the intra-arterial pressure, the injectate can move proximally in the A. angularis. With continuous movement, the injectate can move proximal of the origin of the central retinal artery. If the pressure on the syringe is released, the material moves distally into the retinal artery123–126. Different areas of injection can lead to different means of occlusion. When injecting into the glabellar region, the material moves through the supratrochlear artery followed by the supraorbital artery; in the nasolabial fold any injection in the anastomosis of the dorsal nasal artery from the ophthalmic artery can lead to blindness127.
The usual particle size of HA particles is 400 μm128. This means there is no barrier for the particle to move inside the ophthalmic artery, which has a diameter of about 2 mm, in general. In comparison to that relatively large vessel, the central retinal artery only has a diameter of about 160 μm, a lot smaller than the HA particles, which means it can be easily blocked by HA. Patients suffering from retinal artery occlusion report of immediate blurring or loss of vision and sometimes feel ocular pain.
When suspecting any form of vascular compromise or arterial occlusion the injection of any material should be stopped immediately, and heat and nitroglycerin paste should be applied in order to induce vasodilation until improvement is visible. In addition, at least 75 U of hyaluronidase combined with 1.5 ml lidocaine 0.5%129 should be injected along the distribution of the underlying vessel and the adjacent violaceous skin to dissolve possibly all of the previously injected material and decompress the vessel130. The area should be massaged intensively131 after injection of hyaluronidase. For more severe or unresponsive cases a deep subcutaneous injection of low molecular-weight heparin into the affected area has shown good results with vascular compromise132.
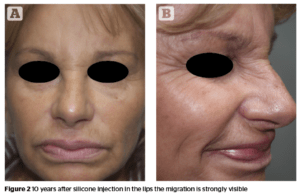
Migration of the product
The more permanent a dermal filler, the higher the risk of migration. This has been well documented, e.g. with polytetrafluorethylene133. Several studies with CaHA showed no case of migration134–136, whereas other reports discuss the migration of CaHA especially in the perioral region causing a ‘popcorn lip’137. This migration of the product may occur even several years after injection. Silicone has a unique feature among all dermal fillers, the ability to migrate to locations distant from the injection site. The clinical presentation has a wide variety of cases and the spread of silicone to many organs has been reported138.
Conclusion
The market for dermal fillers has expanded exponentially in recent years. Dermal fillers nowadays do not only flatten wrinkles, they also restore facial volume and reshape the look of the face. To achieve good looking results, the profound knowledge of the individual and characteristics of the substances used is mandatory. This includes indications, contraindications as well as the prevention and avoiding of any potential side-effects as well as the treatment of any such side-effects, if they should occur.
No filler on the market is without adverse effects, but the appearance can be drastically reduced if the practitioner is familiar with the different products that are needed to achieve the desired results. Almost all severe side-effects are avoidable with profound knowledge of anatomy, planning, and technique.





