Telomere is a biomarker for longevity
Since telomeres undergo attrition with each division of somatic cells in culture, telomere length in somatic cells represents the replicative history, as well as the replicative potential of these cells. Taken together with the long lifespan of humans and their short telomeres, the attrition of telomeres may be a major determinant of human ageing not only at the cellular level, but also at the organ or systemic level. In this context, telomere length of peripheral blood has been successfully used for the evaluation of ageing or perceived age in the cardiovascular system10,11.
Studies suggest the telomere length of white blood cells can be used as a surrogate marker of telomeres for other organs such as vessels, skin, or other tissues. In a French cohort study, Benetos et al10 measured the telomeres of white blood cells from 120 healthy men and 73 healthy women to compare telomere length with pulse pressure, as well as pulse wave length, both of which are the clinical markers of large artery stiffness. In both genders, telomere length was inversely correlated with age (P<0.01), while it was significantly longer in women than in men (8.67±0.09 versus 8.37±0.07 Kb; P=0.016). Multivariate analysis further showed that telomere length significantly contributed to pulse pressure and pulse wave velocity in men, but not in women. This result suggests that men with shorter telomeres are more likely to have stiffened large arteries with atherosclerosis. The data also implied that telomere shortening causes various pathologies associated with age-related diseases such as atherosclerosis.
Telomere measurement also provides a useful biomarker for the survival of twin sisters or brothers. In a twin cohort study in Denmark, Christensen et al11 measured telomere length of white blood cells from 1826 twins aged over 70 years for 10 years to compare telomere length with perceived age. Intriguingly, this study showed that the sister with longer telomeres looked younger and was more likely to survive for a longer time than the sibling bearing shorter telomeres.
Short telomeres and human diseases
On a molecular level, telomere dysfunction is thought to be a consequence of shortening telomeric DNA repeats to the extent that they no longer support a telomere/binding protein complex. Short telomeres trigger a DNA damage response that resembles the response elicited by DNA double-strand breaks. D’Adda di Fagagna et al12 found that senescent human fibroblasts displayed a variety of molecular markers for DNA damage checkpoint response, such as nuclear foci of phosphorylated histone H2AX, 53BP1, MDC1, and NBS1, suggesting that chromosome ends of senescent cells expose uncapped telomeres that directly associate with DNA damage response proteins.
Taken together with the phenotype of cellular senescence, the authors suggest that the shortening of telomeres is a molecular trigger of cellular senescence with concomitant failure of cell cycle progression. Short telomeres are also detected in a number of degenerative disorders, such as idiopathic pulmonary fibrosis and bone marrow failure13. Although these degenerative diseases manifest diverse clinical symptoms, the authors suggest the common underlying molecular mechanisms triggered by shortening of telomeres, comprise ‘the telomere syndromes’ — a single syndrome spectrum defined by short telomere defects.
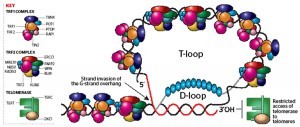
Telomere binding proteins are classified into two categories, TRF1 complex and TRF2 complex according to the binding proteins (Figure 2). Interestingly, mutations found in the genes encoding the binding proteins of TRF2 complex are associated with premature ageing syndromes such as Werner syndrome (WRN), Bloom syndrome (BLM) and Ataxia-telangiectasia-like disorder (ATLD). Homozygous mutations of these genes cause a variety of hereditary premature ageing syndromes with telomere dysfunction, suggesting that the maintenance of telomeres plays an important role in the control of cellular and organismal senescence. The XPF/ERCC1 nuclease is mutated in the human xeroderma pigmentosum syndrome that shows hypersensitivity to UV light, premature ageing and increased incidence of skin cancer.