Peter Christopher Konturek, PhD, MD discusses how the microbial composition of our gut microbiota can be altered through diet, lifestyle and other measures, and the impact this can have on our long-term health and wellbeing

The human intestine is inhabited by a complex microbial community termed gut microbiota. Important members of this community include diverse bacterial strains, viruses, fungi and archaea. The number of gut microbiota is estimated to exceed 1014.1 In other words, the gut microbiota has the largest number of bacteria compared to other parts of the body. Interestingly, the number of microbiota increases along the gut, with the highest amount observed in the large bowel. Among different bacterial phyla, the most important are Firmicutes and Bacteroidetes consisting of more than 90% of all species. Our understanding of the microbial communities that inhabit the human gut has greatly improved in the past decade due to significant biotechnological advances in the metagenomic field2.
The gut microbiota plays an essential role in the host. The most important functions of the gut microbial community include nutrient metabolism and digestion, antimicrobial protection, immunomodulation and enhancement of gut barrier integrity3.
The microbial composition and diversity depend on many factors including genetic and epigenetic factors, mode of delivery, diet, age, exposure to stress, use of drugs (especially antibiotics and proton pump inhibitors), physical exercise, and geographical location4.
The colonisation of gut microbiota occurs during delivery. The mode of delivery and type of nutrition in the first months of life are considered as critically influential factors on early gut microbiota colonization and postnatal development of the intestinal immune system. In the first 3 years of life, the most important part of gut microbiota development takes place. The overuse of antibiotics, false nutrition or exposure to early life stress in that period may have a profound and critical effect on the colonization pattern and diversity of gut microbiota that may influence the occurrence of later diseases5.
Gut Microbiota
Another important and influential factor is the diet that shapes the gut microbiota composition, which has an important effect on gut microbiota function. For example, shifting from a low-fat, high-fibre diet to high-fat, low-fibre diet leads to decreased alpha-diversity (intra-individual gut microbiota richness) and decreased abundance of short-chain fatty acids (SCFA) producing bacteria such as Ruminococcaceae, Lachnospiraceae or Prevotellaceae. Moreover, seasonality, intermittent fasting and circadian rhythmicity are further key factors affecting gut microbiota composition6. Finally, during the ageing process, the composition of gut microbiota changes and the number of so-called pro-inflammatory microbial species involved in ‘silent inflammation’ significantly increases7.
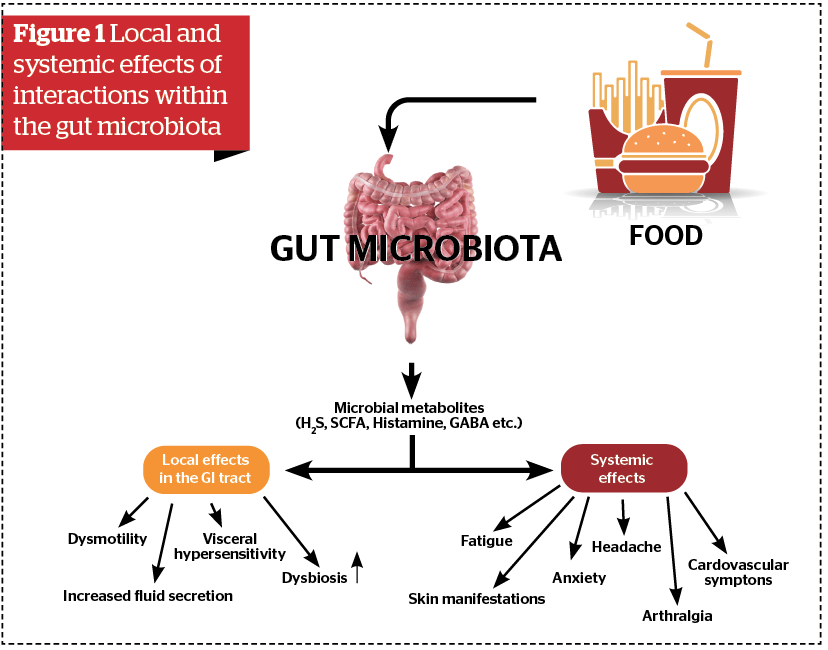
The gut microbiota exists in symbiotic relationships with gut mucosa and fulfils important metabolic, immunological and protective functions in the healthy individual. In other words, the microbiota ecosystem in the gut represents an important arena for host-microbial interactions that have both local and systemic effects (Figure 1).
It has been shown recently that different food compounds may be metabolized to an array of physiologically important metabolites like polyphenol metabolites, tryptophan metabolites or short-chain fatty acids6.
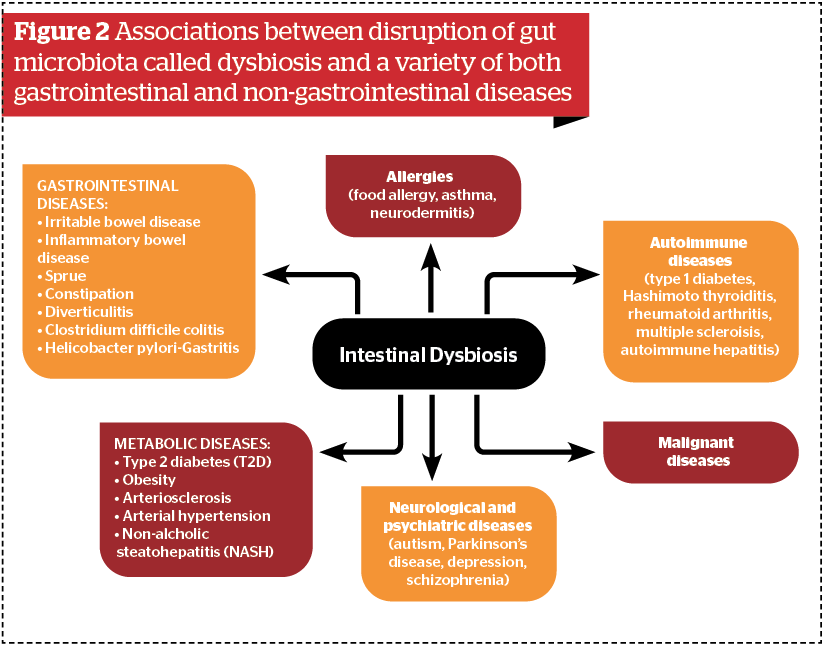
The role of gut microbiota in the promotion of health and development of diseases in and outside the gut has emerged as one of the most important topics of medicine in the 21st century. A number of important associations between disruption of gut microbiota called dysbiosis and a variety of both gastrointestinal and non-gastrointestinal diseases have been observed8 (Figure 2).
Microbial dysbiosis and metabolic diseases
One of the most important associations between intestinal dysbiosis and disease development represent inflammatory bowel diseases (ulcerative colitis and Crohn’s disease). A number of studies have shown a significant disbalance between symbiotic microbiota and pathogenic microbial communities called pathobionts. This results in a significant disturbance to the gut barrier (‘leaky gut’), a break of tolerance to the commensal microbiota and initiation of a chronic inflammatory state in the gut. In the future, it will be important to better characterize microbial signatures and learn more about microbial metabolites that directly influence the function of the immune system in the gut9.
Microbial dysbiosis is also closely associated with the development of functional gut disorders such as gastric dyspepsia or irritable bowel disease. There is strong evidence that gut microbiota affects the function of the so-called gut-brain axis that represents the bidirectional communication between the gut and central nervous system. Gut microbiota and the brain communicate with each other via various routes including the immune system, different microbial metabolites (tryptophan, branched-chain amino acids, LPS and peptidoglycans), the vagus nerve and the enteric nervous system. The disturbance of this microbiota brain-gut axis (MBGA) has been postulated to play an important role in the pathogenesis of functional gut disorders (especially irritable bowel disease). Moreover, the malfunction of MBGA is gaining more interest in the fields investigating the physiological basis of psychiatric, neurodevelopmental, age-related, and neurodegenerative disorders10.
Due to the urbanization of the world, we have witnessed a dramatic increase in the prevalence of metabolic diseases (obesity, type 2 diabetes, hyperlipidemia and arterial hypertension). The typical hallmark of these diseases is a gut microbial dysbiosis, ‘leaky gut’ and the presence of low-grade inflammation (‘silent inflammation’). Obese people, in comparison with lean people, show an increased Firmicutes/Bacteroidetes ratio (F/B-ratio) and decreased microbial diversity. In addition, ‘obese type microbiota’ displays an increased ability to extract more energy from the diet. Interestingly, the colonization of germ-free mice with obese type microbiota caused a greater increase in body fat than the colonization with ‘lean type microbiota’. These experimental observations indicate that the extraction of energy from the diet depends on the composition of gut microbiota and is transmissible. The recent investigations demonstrated that microbiota in obese subjects shows a significant decrease in short-chain fatty acid-producing taxa that affect the release of GLP-1 and ghrelin modulating insulin resistance and the control of food intake11.
Non-alcoholic fatty liver disease is a typical manifestation of metabolic syndrome in the liver. NAFLD encompasses a spectrum of conditions including simple steatosis (accumulation of lipid within hepatocytes), non-alcoholic steatohepatitis, characterized by hepatocellular damage, inflammation and fibrosis, which are significant risk factors for the development of liver cirrhosis and hepatocellular carcinoma. Recent investigations have demonstrated that gut microbial dysbiosis may have an important impact on the progression of steatohepatitis toward fibrosis. Responsible for this phenomenon are different microbial metabolites and particles via the leaky gut that directly or indirectly activate the immune system in the liver. Non-alcoholic steatohepatitis is the fastest-growing cause of hepatocellular carcinoma in liver transplant candidates worldwide12.
Finally, gut microbial dysbiosis has been linked to oncogenesis. The potential involvement of gut microbiota in cancer development involves:
- Induction of chronic inflammatory state by toxins and activation in different signalling pathways
- Breakdown of the normal mucosal barrier and increased release of pro-inflammatory cytokines into circulation
- Increased production of reactive oxygen species
- Impairment of antitumor immune function
- Production of toxins that directly damage DNA (genotoxins)
- Induction of oncogenic transcriptional activity; and
- Disbalance between primary and secondary bile acids.
- Moreover, gut microbiota composition impacts both responses to various cancer therapies (especially immune checkpoint therapy) and associated toxicities13.
The biggest challenge of the current microbiota-based therapies is to change the gut microbiota composition through exogenous administration of beneficial microbes (probiotics) or induce the growth of commensal microbiota through different compounds termed prebiotics. However, the use of prebiotics or probiotics or a combination of both (symbiotics) represents a relatively unspecific approach to manipulate the gut microbiota. Faecal microbiota transfer (FMT) represents a new and more effective method to manipulate gut microbiota. It involves the transfer of the entire microbial community from a healthy donor to a diseased recipient in order to replace the disease-associated microbiota. FMT has been shown to have remarkable efficacy in treating recurrent Clostridium difficile infection (CDI), which is the most common healthcare-associated infectious disease. Currently, FMT is being investigated for other indications, including gastrointestinal and extraintestinal diseases14.
Conclusion
In conclusion, intestinal microbiota plays a crucial role in human health and disease, while gut dysbiosis is associated with various gastrointestinal and nongastrointestinal diseases. The composition of the gut microbiota is influenced by the use of antibiotics and by the lifestyle of the host, including exercise, diet and hygiene preferences. With the increased understanding of the relationship between the human microbiota and a variety of associated diseases, the use of microbiota-based therapies (pre-, pro- and postbiotics, FMT) will gain more importance in the future.
Declaration of interest None
References
- Ventura M, Turroni F, Canchaya C, Vaughan EE, O’Toole PW, van Sinderen D. Microbial diversity in the human intestine and novel insights from metagenomics. Front Biosci (Landmark Ed). 2009 Jan 1;14:3214-21
- Di Bella JM, Bao Y, Gloor GB, Burton JP, Reid G. High throughput sequencing methods and analysis for microbiome research. J Microbiol Methods. 2013 Dec;95(3):401-14
- Cani OD. Human gut microbiome: hopes, threats and promises. Gut 2018 Sep;67(9):1716-1725
- Long-Smith C, O’Riordan KJ, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota–Gut-Brain Axis: New Therapeutic Opportunities. Annu Rev Pharmacol Toxicol. 2019 Sep 10. doi: 10.1146
- Zimmermann P, Curtis N. Factors Influencing the Intestinal Microbiome During the First Year of Life. Pediatr Infect Dis J. 2018 Dec;37(12):e315-e335
- Kolodziejczyk A Zheng D, Elinav E. Diet-microbiota interactions and personalized nutrition. Nat Rev Microbiol. 2019 Dec;17(12):742-753
- Xu C, Zhu H, Qiu P. Aging progression of human gut microbiota. BMC Microbiol. 2019 Oct 28;19(1):236. doi: 10.1186/s12866-019-1616-2
- Durack J, Lynch SV. The gut microbiome: Relationships with disease and opportunities for therapy. J Exp Med. 2019 Jan 7; 216(1): 20–40
- Zuo T, Ng, SC. The Gut Microbiota in the Pathogenesis and Therapeutics of Inflammatory Bowel Disease. Front Microbiol. 2018; 9: 2247. Published online 2018 Sep 25. doi: 10.3389/fmicb.2018.02247
- Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Irritable bowel syndrome: A microbiome-gut-brain axis disorder ?? World J Gastroenterol. 2014 Oct 21; 20(39): 14105–14125
- Arslan N. Obesity, fatty liver disease and intestinal microbiota. World J Gastroenterol. 2014 Nov 28; 20(44): 16452–16462
- Konturek PC, Harsch IA, Konturek K, Schink M, Konturek T, Neurath M, Zopf Y. Gut-–Liver Axis: How Do Gut Bacteria Influence the Liver?? Med Sci (Basel) 2018 Sep; 6(3): 79. Published online 2018 Sep 17. doi: 10.3390/medsci6030079
- Tsilimigras M, Fodor A, Jobin Ch. Carcinogenesis and therapeutics: the microbiota perspective. Nat Microbiol. 2017 Feb 22; 2: 17008. Published online 2017 Feb 22. doi: 10.1038/nmicrobiol.2017.8
- Zeng W, Shen J,Bo T, Peng L, Xu H, Nasser MI, Zhuang Q, Zhao M. Cutting Edge: Probiotics and Fecal Microbiota Transplantation in Immunomodulation. J Immunol Res. 2019; 2019: 1603758. Published online 2019 Apr 16. doi: 10.1155/2019/1603758