Autologous fat grafting and PRP
PRP is a volume of autologous plasma that has a concentration of platelets greater than baseline. The autologous concentration of human platelets in a small volume of plasma is shown in Figure 4. As there is a high concentration of platelets, there is also an increased concentration of the seven fundamental protein growth factors proven to be actively secreted by platelets to initiate wound healing, including (Table 2):
- Three isomers of platelet-derived growth factor (PDGF)
- Two of the numerous transforming growth factors (TGF)
- Vascular endothelial growth factor (VEGF)
- Epithelial growth factor (EGF).
All of these growth factors have been documented to exist in platelets.
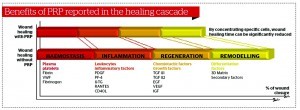
Growth factors are the biologically-active signal peptides released from local tissue or blood products (particularly the platelet fraction), which play a critical role in inducing the initiation and progression of the normal wound healing process. Such factors synchronise the processes of epithelialisation, angiogenesis, and collagen matrix formation, which are the key steps in the wound healing sequence. Growth factors function in paracrine, endocrine and autocrine protocols in order to guide the stages of wound healing. No single growth factor appears to maintain a total physiologic task, but these peptides work in a coordinated fashion to orchestrate the normal wound healing processes. The PRP growth factors never enter the cell or its nucleus, they are not mutagenic, and they act through the stimulation of normal healing, just much faster. Consequently, PRP has no ability to induce tumour formation.
After rinsing the harvested graft material to effectively reduce the intracellular lidocaine concentration and allow the removal of extracellular lipid materials and debris, the PRP is added to the autologous graft materials in an approximate ratio of 5–10% in small volume cases, and 10–20% of the total graft prepared for large volume transplantation.
Growth factors and the three stages of fat graft acceptance
Both PDGF and TGF-β1 are concentrated in the alpha granule of the platelet, and are released with platelet cellular activation and degranulation. In the autologous fat graft, it is convenient to characterise three stages of graft acceptance:
During the first stage, cellular differentiation and activation of preadipocytes begins with the release of PDGF and TGF-β1 during the degranulation of platelets. PDGF is believed to both stimulate mitogenesis and differentiation of the preadipocyte. Simultaneously, angiogenesis (VEGF) is stimulated, resulting in capillary budding and in-growth via induction of endothelial cell mitosis. This initial stage of graft healing is measured in hours and days, with high activity persisting for up to 1 week.
The second stage, typified by fibroblast activation, usually lasts for up to 2 weeks. It is suggested that TGF-β1 is responsible for initial fibroblastic activation, resulting in increased numbers of fibroblasts, and stimulates additional differentiation of precursor adipocyte cells13,17,26,36–38. The prolonged presence of TGF-β1 further acts to stimulate adipocyte lipogenesis, while fibroblasts begin to synthesise pro-collagen type I in preparation for deposit of the collagen matrix to support the capillary in-growth activities. The activated fibroblasts begin synthesis of fibronectin and hyaluronic acid — essential elements of the new extracellular matrix.
The third stage represents the beginning of wound maturation and typically remains active for up to 1 year. During this time, TGF-β1 continues to be active, encouraging additional fibroblast activity. PDGF activates the secretion of certain enzymes (i.e. collagenase) to assist in collagen remodelling and wound maturation processes. The action of these growth factors is clearly synergistic in the promotion of acceptance of autologous fat grafts.
Platelets are the richest known source of PDGF; although it has also been identified within macrophages, fibroblasts, and some endothelial cells. Likewise, TGF-β1 concentrates are primarily released from the alpha granule, but have also been isolated in macrophages. As TGF-β1 is also chemotactic for the recruitment of macrophages and fibroblasts, it is a potent stimulator of granulation tissue formation. Working synergistically, PDGF initially acts to attract monocytes, macrophages, or both, and then becomes an activator to begin production and secretion of additional PDGF into the wound area from macrophages. This is known as an autocrine amplification system, and is important to sustain the wound healing processes.
Benefits of PRP on graft survival
The experience suggests that the use of PRP increases the long-term retention of the transplanted fat cells and increases the rate of revascularisation and survival of the transplanted cells13, 17, 26,36–38.
Results of in vitro studies show that the incorporation of PRP during preparation of autologous fat for transplantation both increases cellular survival and proliferative potential39,40. This is consistent with the clinical observation of higher retention volume in transplantations performed with PRP-enriched fat relative to conventional fat grafts. In addition, clinical experience indicates the addition of PRP is associated with other advantages, including the acceleration of the healing process and in large volume transfers, reduction of spherical calcifications and lipid cyst formation. This has been confirmed by radiographic and ultrasonic visualisation, noted Dr Robert Alexander13, 17, 26,36–38.
This improvement of the healing rate and graft acceptance is thought to decrease the potential for liponecrosis, lipid cyst formation, and the incidence of spherical microcalcifications, particularly within larger volume augmentation (breast and buttock) areas. The potential of enhanced viability and clinical success of using transplanted fat in both small and large volume applications explains the importance of such combinations to promote natural wound healing mechanisms.
Wound healing
Wound healing is initiated in a very complex environment, which is typically started and maintained from elements specifically derived from platelets. Other than the initiation of coagulation processes, the platelets undergo a degranulation process, which releases a complex group of growth factors and cytokines (peptides) essential for wound healing mechanisms.
On the basis of those clinical observations, it is reported that the addition of PRP (and the attendant addition of high concentrations of growth factors and cytokines)increased the retention of the transplanted fat cells, augmented the rate of revascularisation of the grafts, and aided the differentiation of preadipocyte precursor cells into mature adipocytes to further enhance the retained graft volumes. The introduction of such concentrated growth factors during the preparation and transfer phase seems to potentiate wound healing via normal physiologic mechanisms that control cellular recruitment, migration, and differentiation within the recipient sites.
In addition, such additives contribute to the induction and conduction aspects of both the donor and recipient mesenchymal stem cell (undifferentiated) population and in that way, significantly contribute to overall fat graft success.
Conclusions
Many controversies still remain with regard to the use of fat grafting, and especially in the female breast. There is no doubt, however, that using PRP will improve the result. There are also a number of important techniques available to increase the success of autologous fat grafting, including the preparation of the recipient area using carboxytherapy or mesotherapy with PRP; adding PRP to the fat graft; using fat containing more α2 receptors; choosing the correct cannula for harvesting and placement; and good hand–brain coordination.